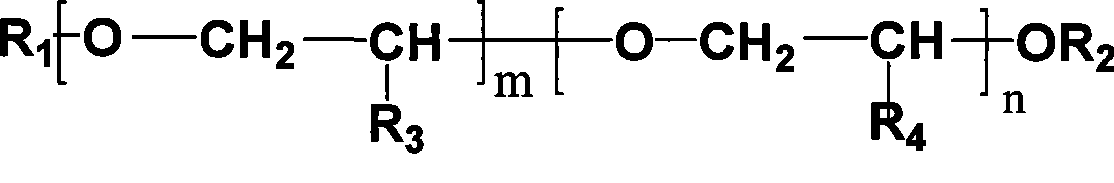Method for synthesizing isosorbide dimethyl ether
A technology of isosorbide dimethyl ether and a synthesis method, which is applied to the synthesis of isosorbide dimethyl ether, and the etherification of isosorbide and methyl chloride to synthesize isosorbide dimethyl ether, can solve the problem of high production cost and achieve The effect of low vapor pressure, shortened reaction time, and reduced production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] In 1L stainless steel autoclave, add 300g molecular weight and be the polyethylene glycol of the methyl termination of 350, 200g (1.37mol) isosorbide, 164g (4.1mol) sodium hydroxide, 2g tetrabutyl ammonium bromide, stir Raise the temperature to 80°C, depressurize and dehydrate with a water pump, the vacuum gauge pressure is -0.095MPa, after the water is removed, feed in methyl chloride, control the pressure at 0.4MPa~0.5MPa, and measure the consumption of methyl chloride, when 208g of methyl chloride After consumption, lower the temperature to 60°C, neutralize to neutral with hydrochloric acid, remove the generated sodium chloride by filtration, conduct vacuum distillation on the filtrate, collect 92°C-93°C / 2mmHg fractions, and obtain 219g of isosorbide dimethyl ether, Yield 92%. 290 g of polyether was recovered.
Embodiment 2
[0042] In 1L stainless steel autoclave, add 300g molecular weight and be the monomethoxy polyethylene glycol of 350, 200g (1.37mol) isosorbide, 216g (5.4mol) sodium hydroxide, 2g tetrabutyl ammonium bromide, stir Raise the temperature to 80°C, depressurize and dehydrate with a water pump, and the vacuum gauge pressure is -0.095MPa. After the water is removed, introduce methyl chloride, control the pressure at 0.4MPa-0.5MPa, and measure the consumption of methyl chloride. When 273g of methyl chloride After consumption, lower the temperature to 60°C, neutralize to neutral with hydrochloric acid, remove the generated sodium chloride by filtration, conduct vacuum distillation on the filtrate, collect fractions at 92°C to 93°C / 2mmHg to obtain 216g of isosorbide dimethyl ether, Yield 91%. 298 g of polyether was recovered.
Embodiment 3
[0044] The reaction conditions are the same as those in Example 1, 250 g of recycled polyether was used as the solvent, and 50 g of fresh polyether was added to obtain 226.5 g of isosorbide dimethyl ether with a yield of 95%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com