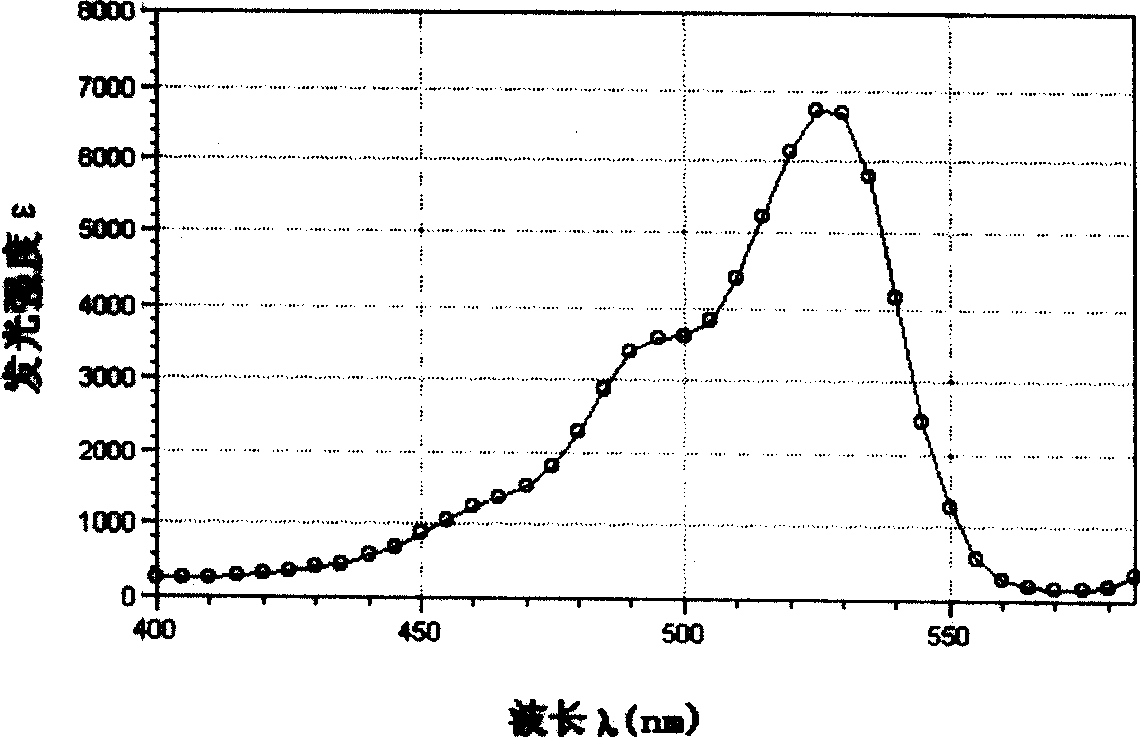
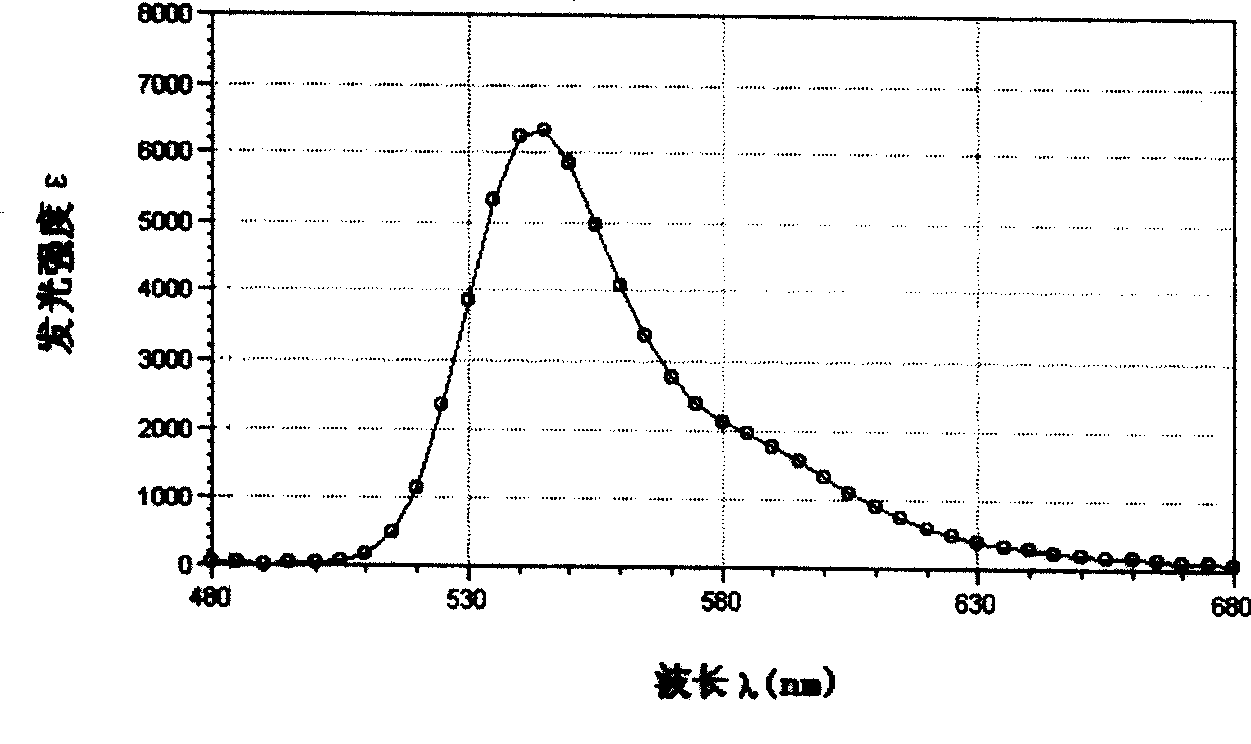
Yellow fluorochrome and synthetic method and use thereof
A technology of yellow fluorescence and synthesis method, applied in luminescent materials, benzoxanthene dyes, chemical instruments and methods, etc., can solve the problems of difficult labeling reagents, difficult selection of excitation wavelength, low fluorescence quantum yield, etc. The effect of large Stokes shift, good fluorescence labeling ability, and high fluorescence quantum yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Embodiment 1: 4,7,2`, the synthesis of 7`-tetrachloro-6-(6-carboxycaproylamido)-fluorescein
[0066] (1) compound 4,7,2`, the synthesis of 7`-tetrachloro-5(6)-carboxyfluorescein, the synthetic steps are as follows:
[0067]
[0068] Add 1.39g (5.33mmol) 3,6-dichlorobenzene triacid anhydride, 1.70g (11.80mmol) 4-chlororesorcinol and 5.00ml concentrated sulfuric acid in a 50mL three-necked flask equipped with a reflux condenser and a drying tube , magnetically stirred, heated up to 190° C. for 24 h, cooled to room temperature, poured the reaction solution into ice-water mixture in batches, and stirred vigorously. A brown-yellow filter cake was obtained by suction filtration, and dried to obtain 2.50 g of a crude product with a yield of 91.20%.
[0069] MALDI-TOF MS, m / z: 515.55 (calcd. 514.09);
[0070] Infrared FT-IR (KBr), υ / cm -1 : 3421, 1759, 1608, 1434, 1212.
[0071] (2) Synthesis of compound 4,7,2`,7`-tetrachloro-5(6)-carboxyfluorescein pivalate
[0072] ...
Embodiment 2
[0104] Example 2: 2', 4, 4', 5', 7, 7'-hexachloro-6-(amidocaproylamido)fluorescein
[0105] (1) Synthesis of compound 2`, 4, 4`, 5`, 7,7`-hexachloro-5(6)-carboxyfluorescein
[0106]
[0107] Take 41.10g (0.23mol) of 2,4-dichlororesorcinol and 26.10g (0.01mol) of 3,6-dichloro-4-carboxyphthalic anhydride into a 500mL three-necked flask, add 200mL of concentrated sulfuric acid, The reaction was carried out at 170° C. for 36 hours under the protection of nitrogen. After the reaction was completed, the reaction mixture was poured into ice water, and a large amount of brick red precipitates were precipitated. After suction filtration, a pink solid was obtained with a yield of 75.00%.
[0108] MALDI-TOF MS, m / z: 581.90 (Calcd: 582.99);
[0109] Infrared FT-IR (KBr), υ / cm -1 : 3480, 1768, 1709, 1478, 1433.
[0110] (2) Synthetic compound of 2`, 4, 4`, 5`, 7,7`-hexachloro-5(6)-carboxyfluorescein pivalate
[0111]
[0112] Take 9.00g (15.00mmol) of 2`, 4,4`, 5`,7,7`-hexachlor...
Embodiment 3
[0140]Example 3: Synthesis of compound 4,7-dichloro-2`, 7`-difluoro-6-(6-carboxyhexanoyl)-fluorescein
[0141] (1) The synthesis of compound 4,7-dichloro-2`,7`-difluoro-5(6)-carboxyfluorescein is similar to Example 1 step (1), except that 2-fluororesorcinol Instead of 2-chlororesorcinol, the yield is 70.00%.
[0142] (2) The synthesis of compound 4,7-dichloro-2',7'-difluoro-5(6)-carboxyfluorescein pivalate is similar to step (2) of Example 1.
[0143] (3) Compound 4, the synthesis of 7-dichloro-2`, 7`-difluoro-tetrachloro-6-carboxyfluorescein pivalate diisopropylammonium salt is similar to Example 1 step (3), producing Rate 35.50%.
[0144] (4) The synthesis of compound 4,7-dichloro-2',7'-difluoro-6-carboxyfluorescein pivalate was similar to step (4) of Example 1, with a yield of 90.00%.
[0145] (5) Compound 4,7-dichloro-2`, 7`-difluoro-di-tert-valeryl-6-carboxyfluorescein-N-hydroxyl succinimidyl carbonate synthesis and embodiment 1 step (5 ) similar, yield 82.60%.
[01...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com