Salt of phenoxypyridine derivative or crystal thereof and process for producing the same
A technology of pyridine and oxygen, which is applied in the field of salts of phenoxypyridine derivatives and their crystallization and preparation, and can solve problems such as undisclosed or implied compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
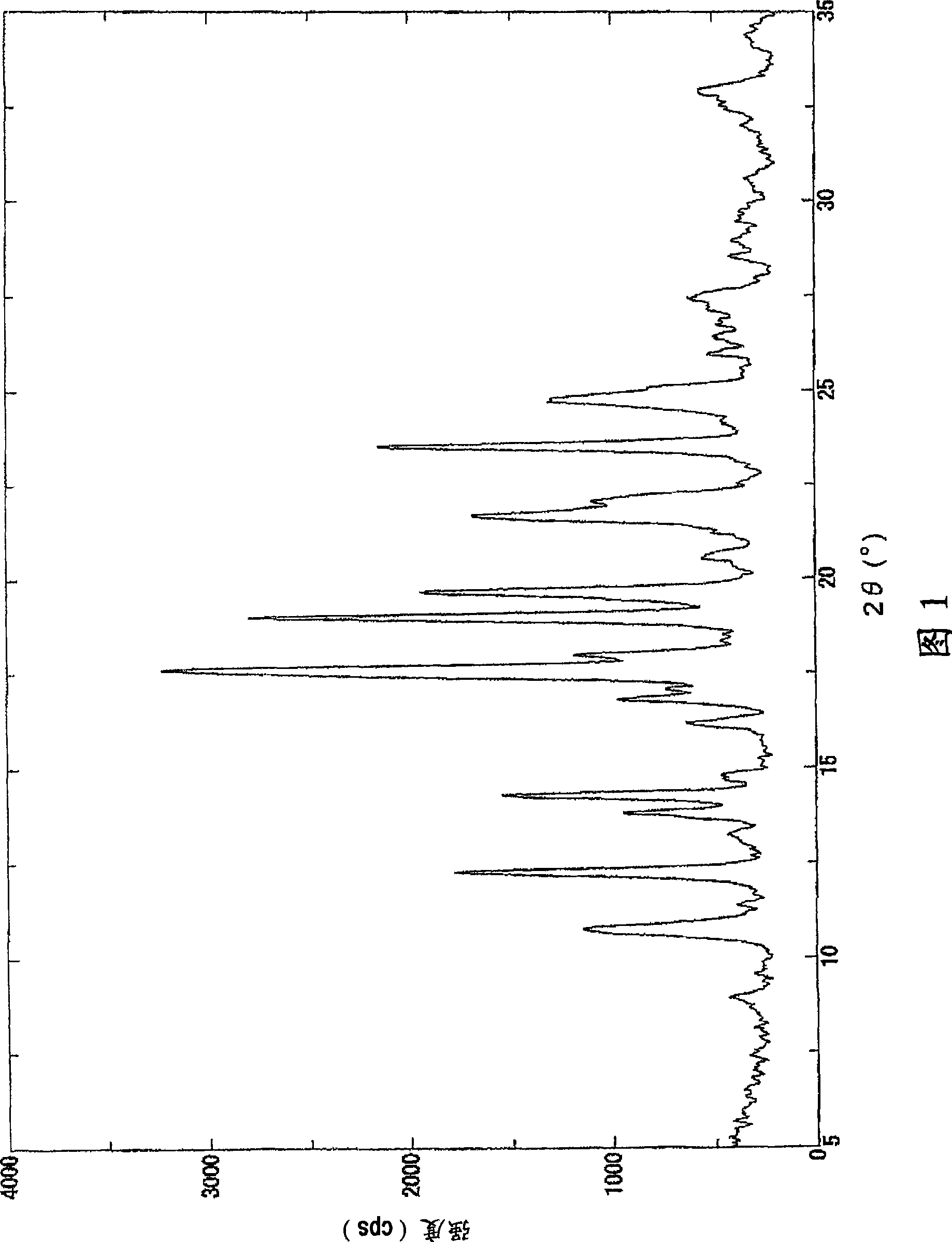
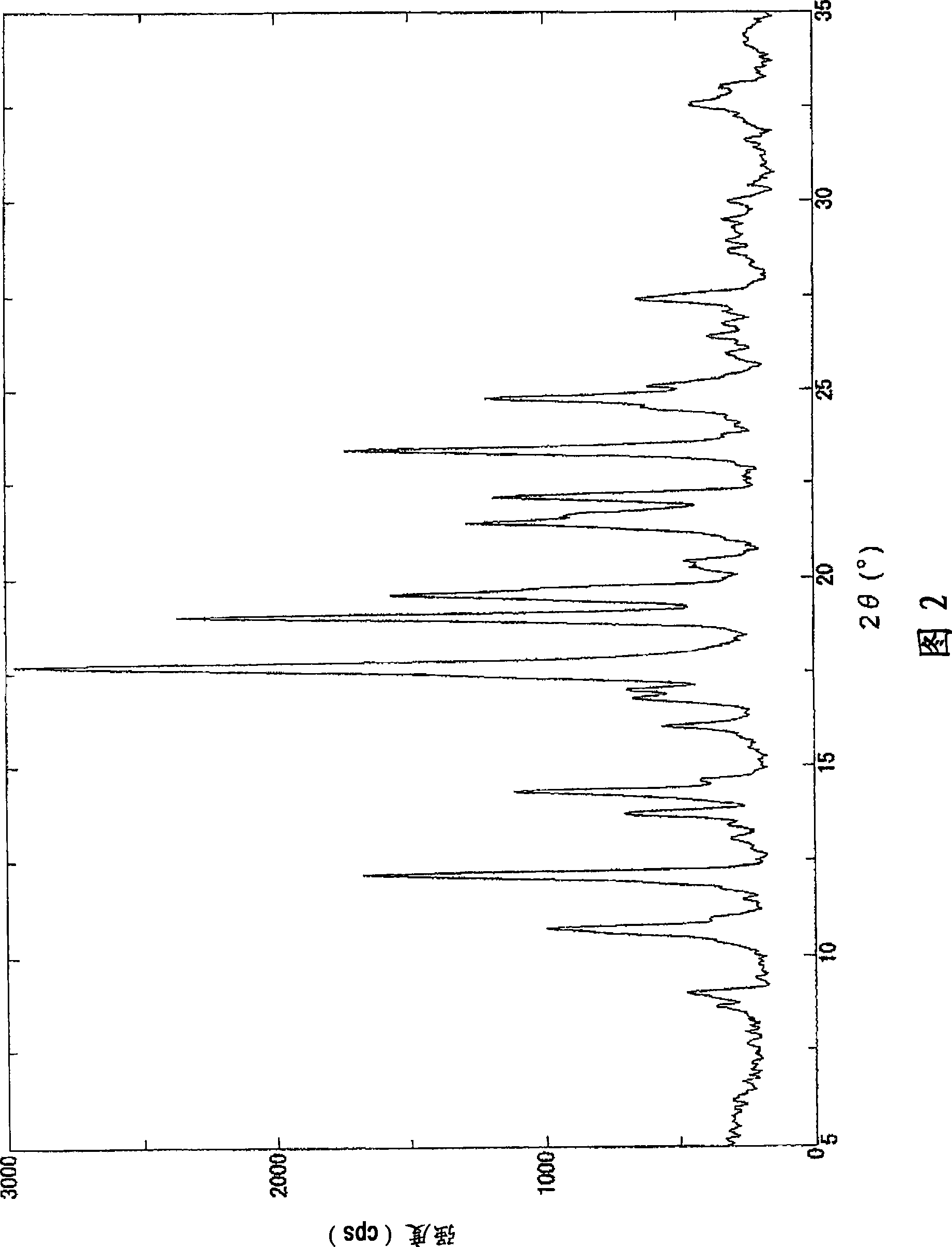
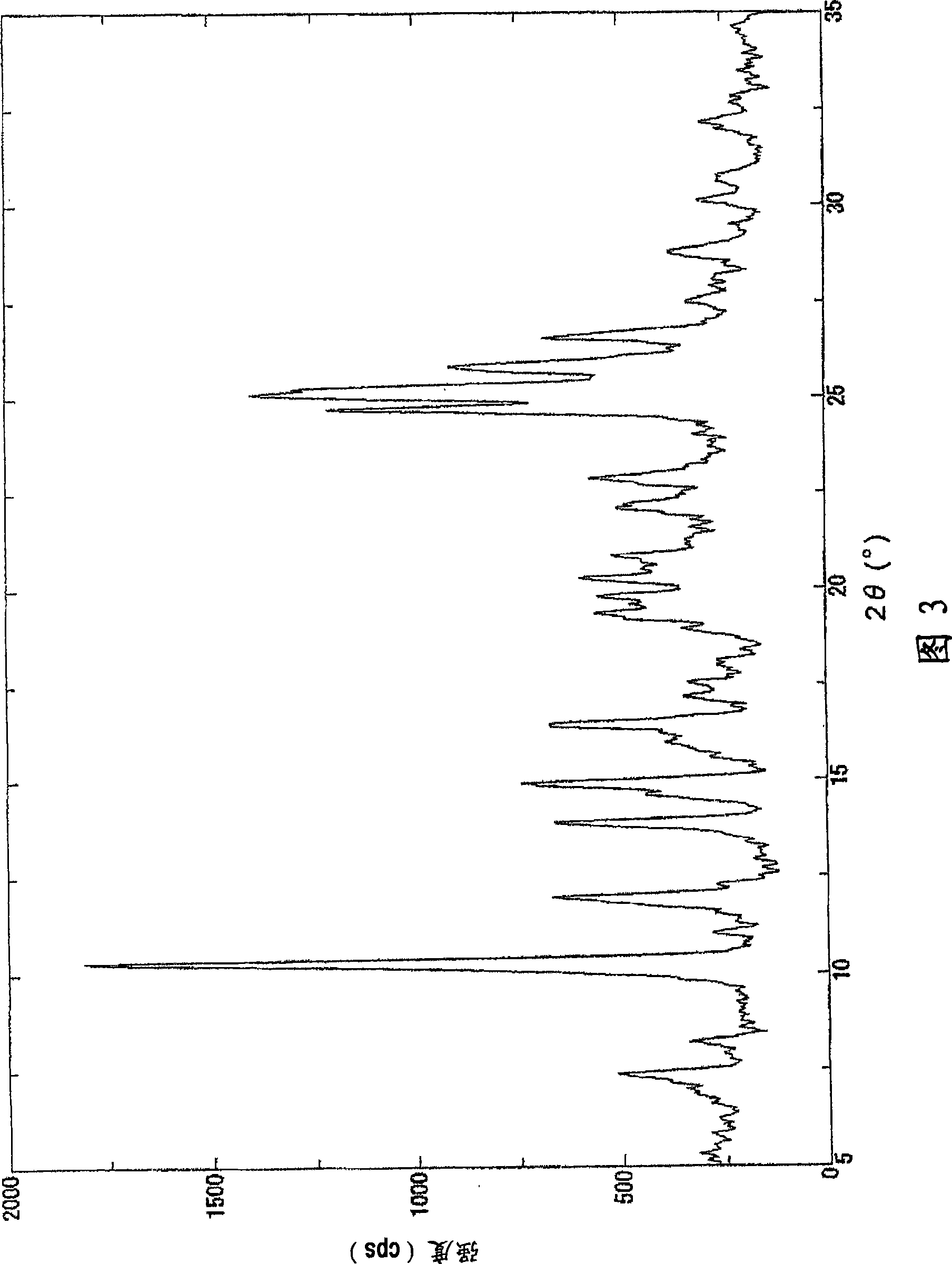
Image
Examples
preparation example Construction
[0074] 1. The preparation method of the crystallization of the malate of compound 1
[0075] Compound 1, a solvent, and malic acid are mixed to form a solution, and crystals are precipitated to prepare crystals of a malate salt of Compound 1.
[0076] More specifically, for example, compound 1 and a solvent are mixed at room temperature, malic acid is added at room temperature or under heating to form a solution, and the solution is gradually cooled to about 4°C to room temperature to precipitate crystals, thereby being able to Crystals of the malate salt of compound 1 were prepared. In addition, it is preferable to stir a solution while gradually cooling.
[0077] As the solvent, for example, ketones such as acetone, alcohols such as ethanol, 1-propanol, and 2-propanol can be used, and ethanol is preferable.
[0078]The amount of solvent is not particularly limited, and it is preferable to use 5 to 30 times the amount of compound 1.
[0079] The amount of malic acid can b...
preparation example 1-1
[0123] (Preparation example 1-1) ethyl 4-chloropyridine-2-carboxylate
[0124] A mixture of 4-chloropyridine-2-carboxylic acid (39.4 g) and thionyl chloride (64 ml) was stirred with heating at 100° C. for 6 hours under a nitrogen atmosphere. The reaction solution was cooled to room temperature. It was concentrated under reduced pressure and azeotroped in toluene. The residue was added a little at a time to ice-cooled and stirred ethanol. The reaction was stirred at room temperature for 25.5 hours. The reaction solution was concentrated under reduced pressure. To the residue was added saturated aqueous sodium bicarbonate solution, which was extracted with ethyl acetate. The organic layer was washed with saturated brine, and dried over anhydrous sodium sulfate. The dried organic layer was concentrated under reduced pressure to obtain the title compound (38.8 g, 83.6%) as a brown oil.
[0125] 1 H-NMR spectrum (CDCl 3 )δ (ppm): 1.46 (3H, t, J = 7.2Hz), 4.50 (2H, q, J = ...
preparation example 1-2
[0126] (Preparation example 1-2) ethyl 4-(3-fluoro-4-nitrophenoxy)pyridine-2-carboxylate
[0127]3-Fluoro-4-nitrophenol (24.7 g) and chlorobenzene (7.0 ml) were added to ethyl 4-chloropyridine-2-carboxylate (19.4 g), and it was heated at 120° C. under nitrogen atmosphere Stir for 4 hours. The reaction solution was cooled to room temperature. Ethyl acetate (400 ml) and saturated aqueous sodium carbonate solution (400 ml) were added thereto, and it was stirred at room temperature for 27 hours. Stirring was stopped and the aqueous layer was separated. Further, saturated aqueous sodium carbonate solution was added to the organic layer, which was stirred at room temperature for 2 days. Stirring was stopped and the aqueous layer was separated. The aqueous layer was extracted with ethyl acetate (300ml). The organic layers were combined and washed with saturated brine. The organic layer was dried over anhydrous sodium sulfate, which was concentrated under reduced pressure. Th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com