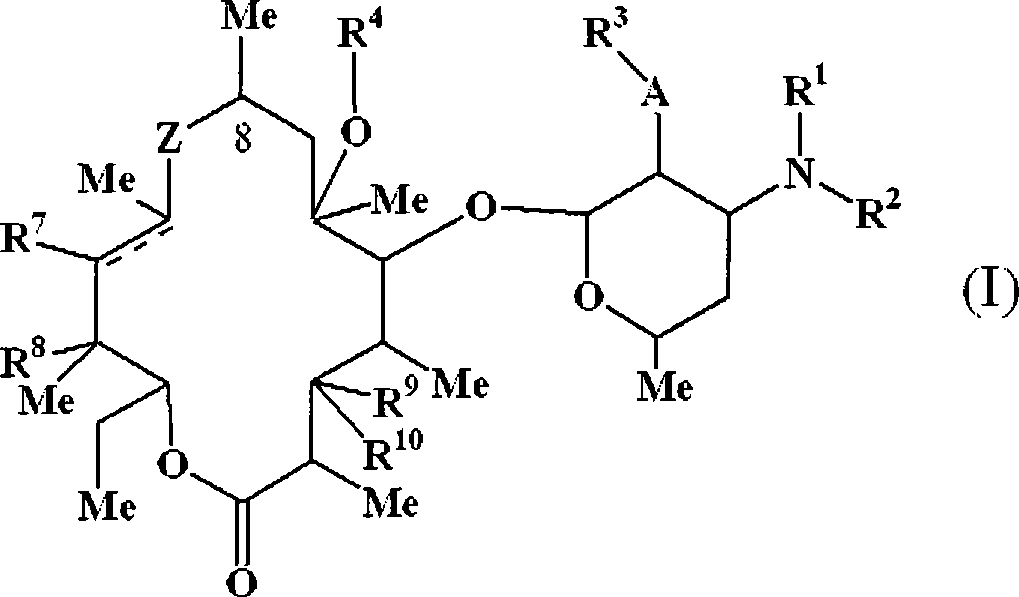
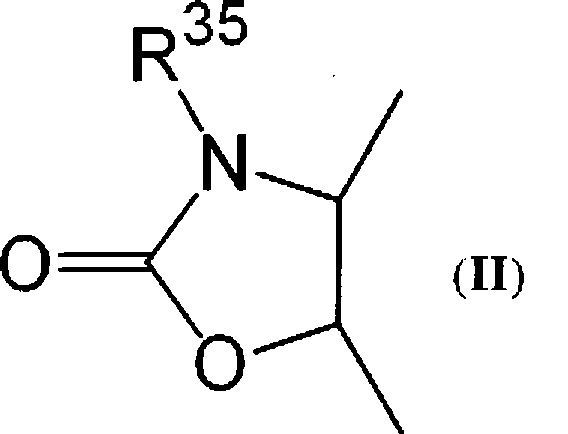
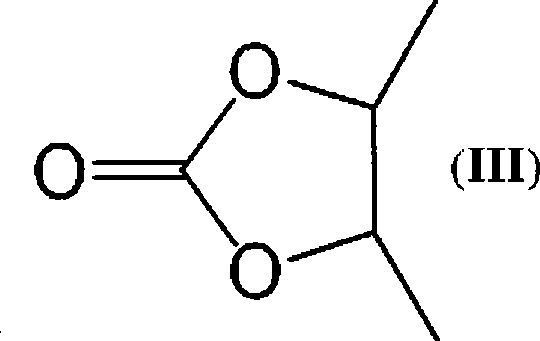
Macrolide derivatives
A compound and ring structure technology, applied in the field of salt and hydrate, can solve problems such as separation without inhibition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0561] 2’-O-(3-oxobut-1-enyl)-9-deoxo-9a-aza-9a-methyl-9a-homoerythromycin A (Homoerisromyishin A)
[0562] Dissolve 1.0 g of 9-deoxo-9a-aza-9a-methyl-9a-homoerythromycin A in 4 ml of chloroform, add 136 μl of 3-butyn-2-one, and stir at room temperature for 15 hours. The reaction solution was distilled off under reduced pressure, and the resulting residue was purified by silica gel chromatography (chloroform:methanol:ammonia water=49:1:0.1 to 24:1:0.1) to obtain 717 mg of the title compound.
[0563] MS(ESI)m / z=817.7[M+H] +
[0564] 13C NMR (126MHz, CHLOROFORM-d) δ ppm 8.72, 11.33, 14.75, 16.22, 18.38, 21.26, 21.72, 22.19, 26.75, 27.65, 32.08, 34.80, 36.41, 40.93, 42.01, 45.44, 45.372, 69 , 68.35, 70.05, 73.17, 74.35, 77.30, 77.51, 78.21, 83.22, 83.92, 94.52, 100.69, 108.54, 164.85, 197.54
Embodiment 2
[0566] 11-Amino-11-deoxy-2’-O-(3-oxobut-1-enyl)-6-O-methylerythromycin A 11,12-cyclocarbamate
[0567] 11-amino-11-deoxy-6-O-methylerythromycin A11,12-cyclocarbamate described in literature (Journal of Organic Chemistry, 1988, volume 53, number 10, pages 2340-2345) 500 mg was reacted in the same manner as in Example 1 to obtain 330 mg of the title compound.
[0568] MS(ESI)m / z=839.5[M+H] +
[0569] 13C NMR (126MHz, CHLOROFORM-d) δ ppm 8.76, 10.55, 13.51, 13.77, 15.74, 18.14, 18.75, 19.94, 21.3, 21.60, 22.08, 28.09, 34.81, 37.61, 39.43, 45.14, 40.8, 34, 45. , 50.12, 57.86, 63.33, 65.96, 68.45, 72.91, 75.89, 77.52, 77.84, 78.48, 80.40, 84.0, 95.68, 100.68, 158.44, 176.83, 197.60, 218.06
Embodiment 3
[0571] 11-Amino-11-deoxy-2’-O-(2-methoxycarbonylvinyl)-6-O-methylerythromycin A 11,12-cyclocarbamate
[0572]Use 500 mg of 11-amino-11-deoxy-6-O-methylerythromycin A11,12-cyclocarbamate, and use 70 μl of methyl propiolate (Mechel propiolet) instead of 3-butyne -2-Kone was reacted in the same manner as in Example 1 to obtain 452 mg of the title compound.
[0573] MS(ESI)m / z=857.6[M+H] +
[0574] 1H NMR (600MHz, CHLOROFORM-d) δ ppm 0.85 (t, J = 7.57Hz, 3H) 0.89 (d, J = 7.79Hz, 3H) 1.10-1.15 (m, 6H) 1.19 (d, J = 7.34Hz, - 1.79 (m, 6H) 1.84-1.92 (m, 1H) 2.13-2.17 (m, 1H) 2.24-2.36 (m, 1H) 2.30 (s, 6H) 2.49-2.58 (m, 1H) 2.65-2.78 (m, 2H)2.81-2.87(m,1H)2.91(s,3H)2.99-3.05(m,1H)3.32(s,3H)3.45-3.51(m,1H)3.52-3.57(m,1H)3.57-3.61( m, 1H) 3.64-3.67 (m, 1H) 3.69 (s, 3H) 3.73-3.81 (m, 1H) 3.88-3.95 (m, 1H) 4.49-4.53 (m, 1H) 4.86-4.90 (m, 1H) 5.04-5.08 (m, 1H) 5.27 (d, J = 11.92Hz, 1H) 5.77 (s, 1H) 7.47 (d, J = 12.38Hz, 1H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com