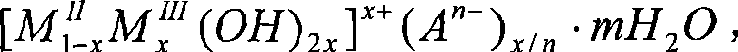Method for removing bromate ion in drinking water
A bromate, drinking water technology, applied in chemical instruments and methods, ion exchange water/sewage treatment, water/sewage treatment and other directions, can solve the problems of many influences, increased chromaticity of effluent, unstable effect, etc., and achieves stability good, low solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
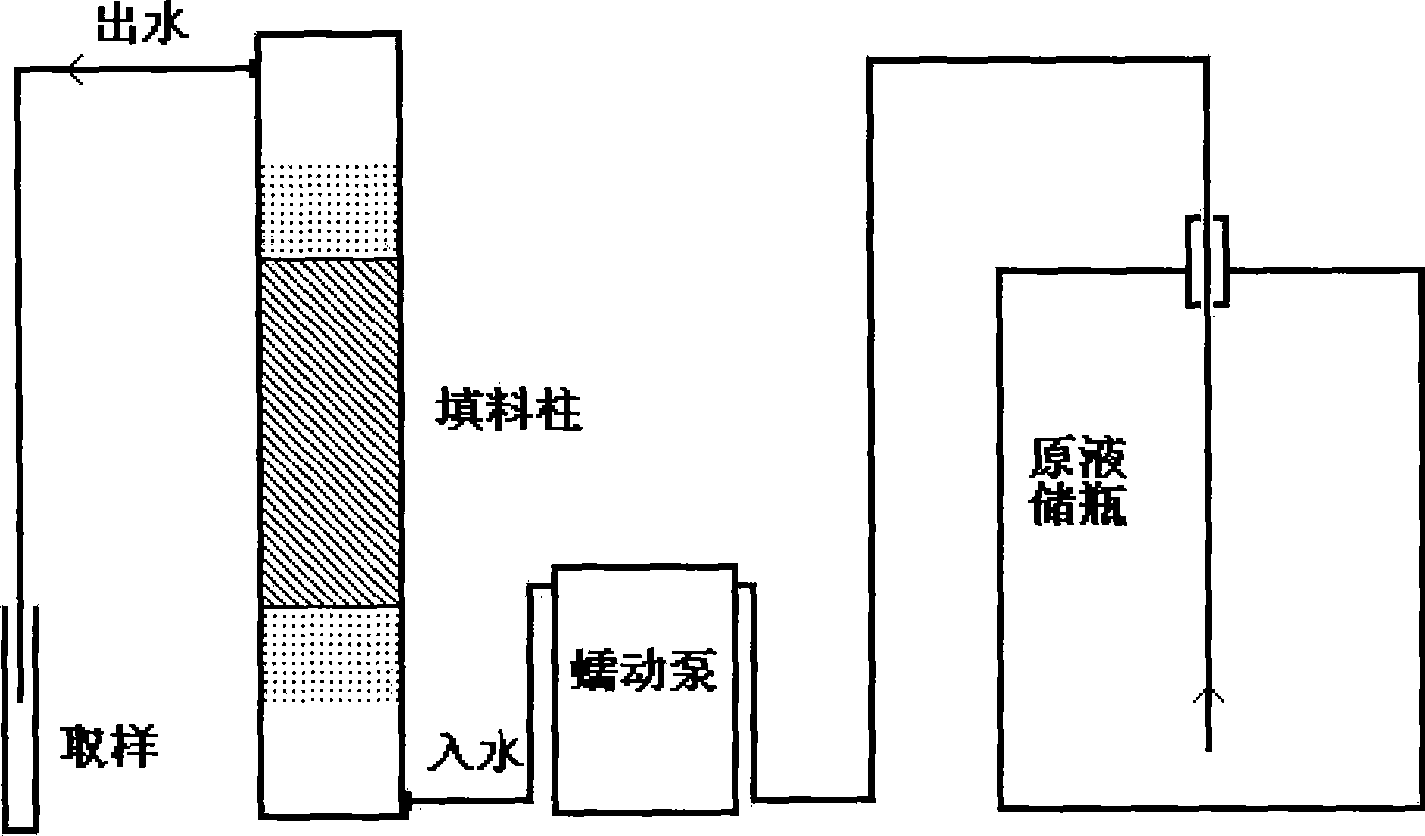
[0018] Weigh 168g of calcium oxide and 102g of alumina, press the mixed solid into a sheet or column, and sinter at 1500°C for 12 hours. The sintered product was ground and then put into a nearly saturated calcium chloride solution, stirred by magnetic force for 48 hours and then filtered. The solid material was dried at 105° C. and then ground. Take 20g of the prepared solid material and fill it into a cylindrical packed column, pass drinking water containing bromate 37.678μg / L from the bottom of the column, and pass through the packed column at a flow rate of 60 ~ 100ml / h and then from the packed column top out. The results showed that after 100L of bromate-containing drinking water was treated, the removal rate of bromate reached 91%-95%, and its concentration dropped below 10μg / L.
Embodiment 2
[0020] Weigh 84g of calcium oxide and 107g of iron hydroxide, press the mixed solid into a sheet or column, and sinter at 1500°C for 12 hours. The sintered product was ground and put into a nearly saturated calcium chloride solution, stirred by magnetic force for 48 hours and then filtered. The solid material was dried at 105° C. and then ground. Get the prepared solid material 10g and drop into 100L drinking water containing bromate 34.532μg / L. The mixture was stirred for 1 hour and then filtered. The results showed that after 100L of bromate-containing drinking water was treated, the removal rate of bromate reached 87%, and its concentration dropped below 10μg / L.
[0021] The solid material referred to in embodiment 1 is the inorganic layered compound Ca-Al-LDH (LDH represents containing OH - The interlayer compound, the original text of LDH is Layered Double Hydroxides).
[0022] The solid material indicated in embodiment 2 is the inorganic layered compound Ca-Fe-LDH (LD...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com