Method for preparing magnesia, silicon dioxide and nickel oxide products from lateritic nickel ore
A technology of laterite nickel ore and silicon dioxide, applied in the directions of silicon dioxide, magnesium oxide, silicon oxide, etc., can solve the problems of long production cycle, increased acid consumption, and high cost of microbial culture, and achieves simple equipment, low cost, and high technology. Simple process effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
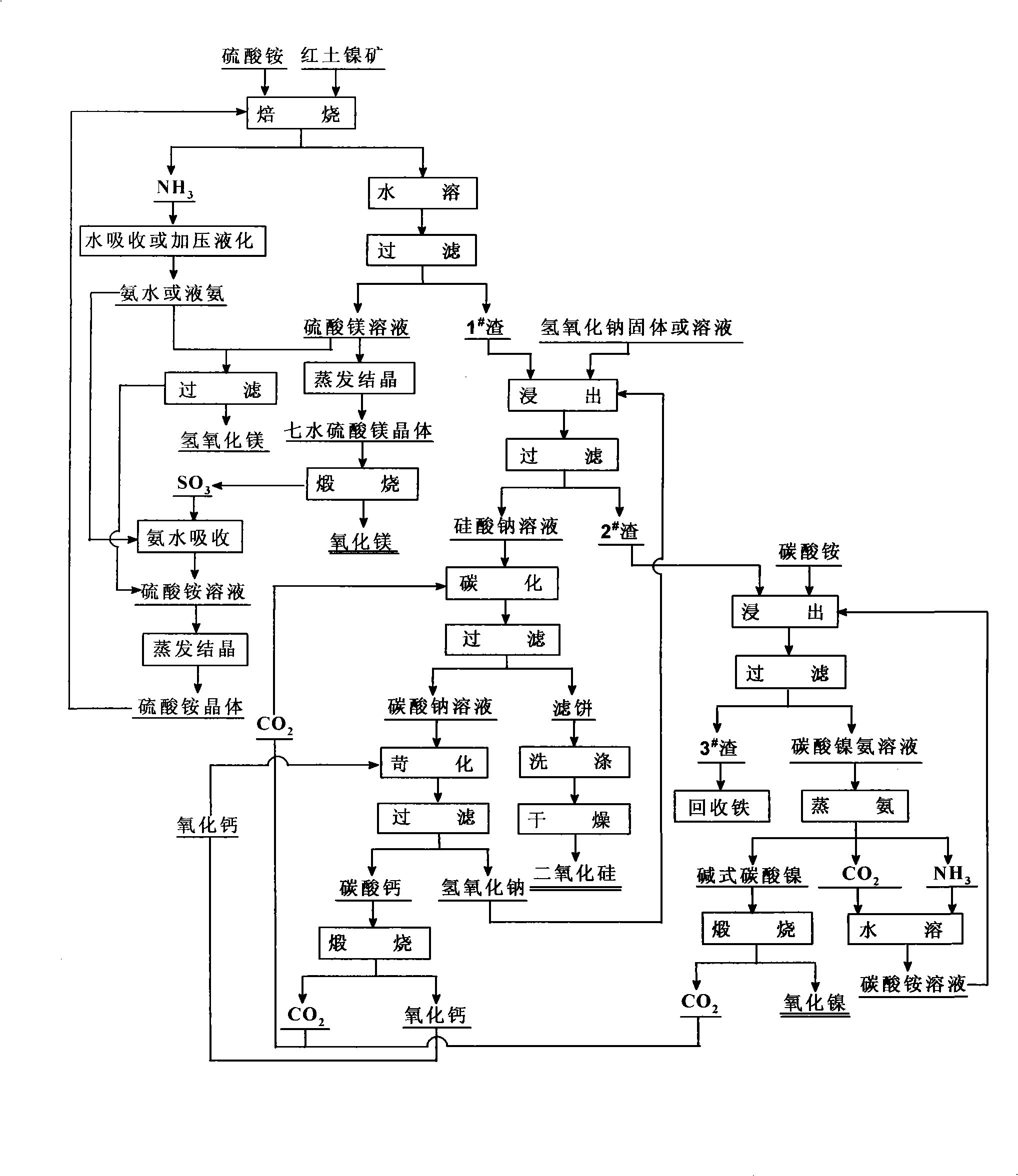
[0037] The composition of the laterite nickel ore used is: NiO 0.93%, SiO 2 40.74%, MgO 21.53%, Fe 2 o 3 18.82%, Al 2 o 3 4.45%, CaO 0.62%, Cr 2 o 3 0.56%, other impurities 0.72%, loss on ignition 11.63%.
[0038] The laterite nickel ore that has been crushed and ground to less than 80 μm is mixed evenly with ammonium sulfate at a molar ratio of 1:3, kept at 500°C for 5 hours, and roasted. The gas released during the reaction is absorbed by water. The roasted product is cooled, dissolved in water, and then separated into solid and liquid. Filtrate is magnesium sulfate solution, and filter residue is 1 # scum.
[0039] Evaporate and concentrate the magnesium sulfate solution to obtain magnesium sulfate heptahydrate. Magnesium sulfate heptahydrate is dehydrated at 300°C for 3 hours, and then heated to 1200°C for 5 hours to obtain magnesium oxide. After inspection, it reaches the HG / T 2573-94 industrial magnesium oxide standard.
[0040] Will 1 # Mix the slag and so...
Embodiment 2
[0045] The composition of the laterite nickel ore used is: NiO 1.32%, SiO 2 37.88%, MgO 23.61%, Fe 2 o 3 19.88%, Al 2 o 3 4.95%, CaO 0.58%, Cr 2 o 3 0.62%, other impurities 0.52%, loss on ignition 10.64%.
[0046] The laterite nickel ore that has been crushed and ground to less than 80 μm is mixed evenly with ammonium sulfate at a molar ratio of 1:4, kept at 450°C for 6 hours, and roasted. The gas released during the reaction is absorbed by water. The roasted product is cooled, dissolved in water, and then separated into solid and liquid. Filtrate is magnesium sulfate solution, and filter residue is 1 # scum.
[0047] Evaporate and concentrate the magnesium sulfate solution to obtain magnesium sulfate heptahydrate, dehydrate the magnesium sulfate heptahydrate at 400°C for 2 hours, then raise the temperature to 1300°C and calcinate for 4 hours to obtain magnesium oxide. After inspection, it reaches the HG / T 2573-94 industrial magnesium oxide standard.
[0048] Will...
Embodiment 3
[0053] The composition of the laterite nickel ore used is: NiO 1.73%, SiO 2 42.57%, MgO 20.31%, Fe 2 o 3 18.66%, Al 2 o 3 3.87%, CaO 0.68%, Cr 2 o 3 0.52%, other impurities 0.86%, loss on ignition 10.8%.
[0054] The laterite nickel ore that has been crushed and ground to less than 80 μm is mixed evenly with ammonium sulfate at a molar ratio of 1:5, kept at 600°C for 4 hours, and roasted. The gas released during the reaction is absorbed by water. The roasted product is cooled, dissolved in water, and then separated into solid and liquid. Filtrate is magnesium sulfate solution, and filter residue is 1 # scum.
[0055] Evaporate and concentrate the magnesium sulfate solution to obtain magnesium sulfate heptahydrate, dehydrate the magnesium sulfate heptahydrate at 500°C for 2 hours, and then heat up to 1400°C for 3 hours to obtain magnesium oxide. After inspection, it reaches the HG / T 2573-94 industrial magnesium oxide standard.
[0056] Will 1 # The slag and the so...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com