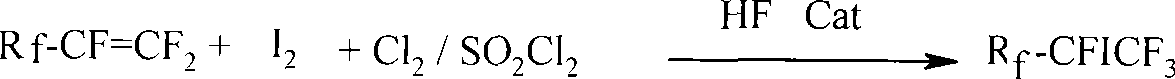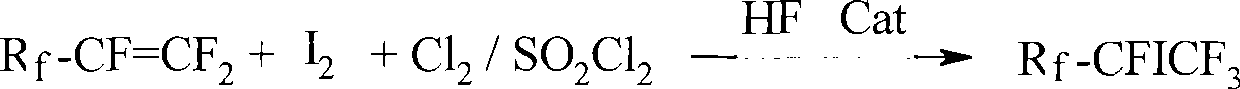Method for preparing single iodo perfluoro alkane
A technology of perfluoroalkane and iodide, which is applied in the field of preparation of small molecule monoiodoperfluoroalkane, can solve the problems of cumbersome operation, high price and low yield in the reaction process, and achieve reduced production cost, convenient operation, and high yield. rate-enhancing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Pass high-purity nitrogen into a 500 ml stainless steel autoclave with electromagnetic stirring to remove the oxygen in the autoclave. After testing to confirm that the autoclave is oxygen-free, add 122g (0.48mol) of iodine and 67.5g (0.5mol) of sulfonyl chloride , 0.68 g (0.01 mol) of boron trifluoride, 100 g (5 mol) of anhydrous hydrofluoric acid and 40 g (0.4 mol) of tetrafluoroethylene. Slowly heat under stirring, raise the temperature to 140±2°C within 1 hour, keep the temperature and continue to react for 5 hours, the reaction product is naturally cooled to 50°C, and the released gas phase mixture passes through the absorption tank of 10wt% sodium hydroxide aqueous solution, and then passes through the A dry bottle of calcium chloride-like calcium chloride, then absorbed in a cold well at -78°C to obtain the crude pentafluoroiodoethane, and the crude product was distilled at low temperature to collect fractions with a boiling point of 12-13°C to obtain 94.4g of pen...
Embodiment 2
[0025] Feed high-purity nitrogen into a 500 ml stainless steel autoclave with electromagnetic stirring to remove oxygen in the autoclave. After testing to confirm that the autoclave is oxygen-free, add 127g (0.5mol) of iodine and 67.5g (0.5mol) of sulfonyl chloride , 13.8g (0.05mol) of tantalum pentafluoride, 200g (10mol) of anhydrous hydrofluoric acid and 40g (0.4mol) of tetrafluoroethylene. Slowly heat under stirring, raise the temperature to 180±2°C within 1 hour, keep the temperature and continue to react for 3 hours, the reaction product is naturally cooled to 50°C, the released gas phase mixture passes through the absorption tank of 10wt% sodium hydroxide aqueous solution, and then passes through the A dry bottle of calcium chloride-like calcium chloride, and then absorbed in a cold well at -78°C to obtain a crude pentafluoroiodoethane. The crude product was distilled at low temperature to collect fractions with a boiling point of 12-13°C to obtain 90.5 g of pentafluoroio...
Embodiment 3
[0027] Feed high-purity nitrogen into a 500 ml stainless steel autoclave with electromagnetic stirring to remove oxygen in the autoclave. After testing to confirm that the autoclave is oxygen-free, add 127g (0.5mol) of iodine and 67.5g (0.5mol) of sulfonyl chloride , 33.1 g (0.08 mol) of aluminum triiodide, 200 g (10 mol) of anhydrous hydrofluoric acid and 40 g (0.4 mol) of tetrafluoroethylene. Slowly heat under stirring, raise the temperature to 160±2°C within 1 hour, keep the temperature and continue to react for 7 hours, the reaction product is naturally cooled to 50°C, the released gas phase mixture passes through the absorption tank of 10wt% sodium hydroxide aqueous solution, and then passes through the A dry bottle of calcium chloride-like calcium chloride, then absorbed in a cold well at -78°C to obtain the crude product of pentafluoroiodoethane, and the crude product was distilled at low temperature to collect the fraction with a boiling point of 12-13°C to obtain 91.5g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com