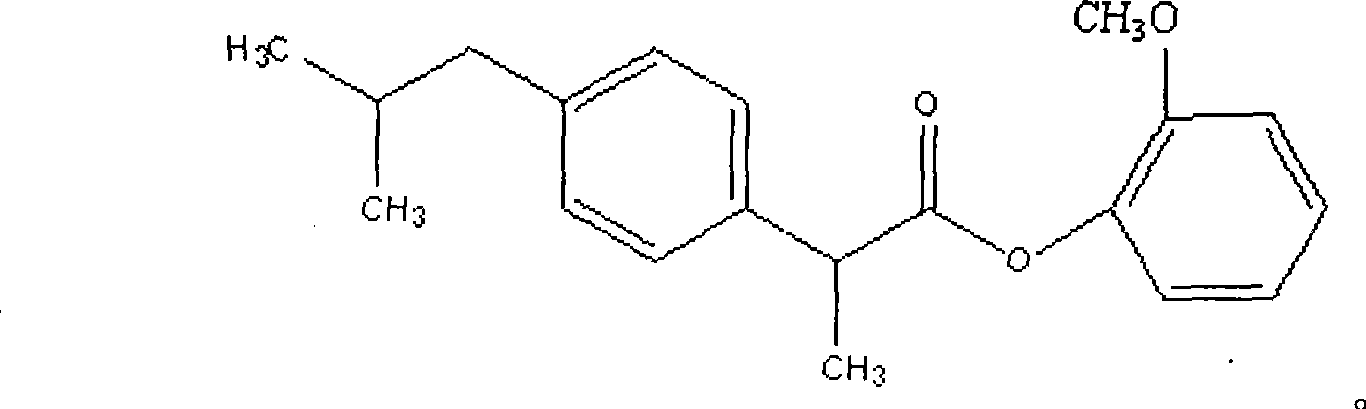
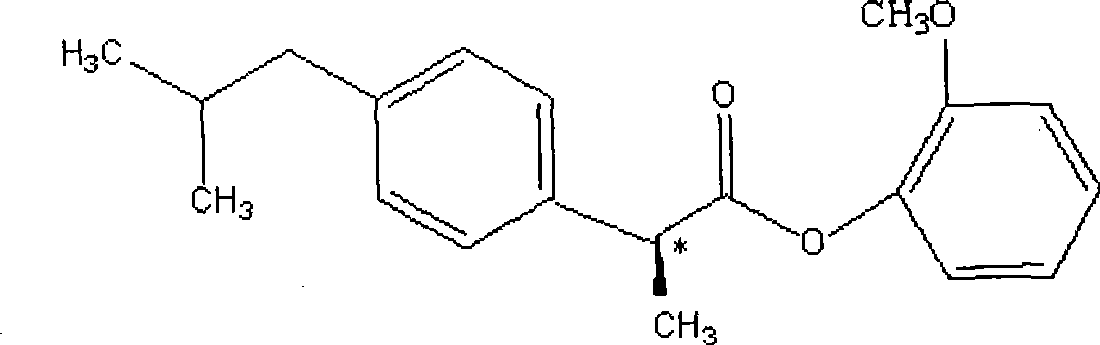
Dexibuprofen guaiacol ester and preparation method thereof
A technology of guaiacol and lignan ester, which is applied in the field of western medicine invention, can solve the problems of enantiomers having no drug effect and harmfulness, and achieve the effects of relieving pain, overcoming liver damage, and benefiting drug dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Add 20.5g (0.1mol) of dextroibuprofen and 20ml of thionyl chloride into a 100ml dry single-necked bottle, heat (control the temperature at 60°C) and stir, and reflux until no hydrogen chloride gas is generated, then evaporate at normal pressure Excessive thionyl chloride gave 20.2 g (+) α-methyl-4-(2-methylpropyl) phenylacetyl chloride as a pale yellow liquid with a strong pungent odor. Add 18g (0.08mol) (+) α-methyl-4-(2-methylpropyl) phenylacetyl chloride and 9ml (0.08mol) guaiacol of the above-mentioned obtained liquid to a 100ml dry single-mouth bottle, add 60ml of dichloromethane was cooled in an ice-water bath and 10ml of triethylamine was added dropwise under strong mechanical stirring, and the raw material guaiacol basically reacted completely as detected by TLC. The reaction time is about 5 hours. The reaction system was washed with water until neutral, then dried by adding anhydrous sodium sulfate, the solvent was recovered under reduced pressure to obtain an...
Embodiment 2
[0034]Add 10g (0.049mol) dextroibuprofen and 10ml phosphorus trichloride to a 50ml dry single-necked bottle, heat (control the temperature at 30°C) and stir and reflux until no hydrogen chloride gas is generated, then evaporate excess trichloride under normal pressure Phosphate, to obtain light yellow liquid 8g (+) α-methyl-4-(2-methylpropyl) phenylacetyl chloride with a strong pungent odor. Then add 6g (0.027mol) (+) α-methyl-4-(2-methylpropyl) phenylacetyl chloride and 3ml (0.027mol) guaiacol of the above-mentioned obtained liquid into a 100ml dry single-necked bottle, add 30ml of acetone was cooled in an ice-water bath and 40ml of 5% sodium hydroxide solution was added dropwise under strong mechanical stirring, and the raw material guaiacol basically reacted completely as detected by TLC. The reaction time is about 4 hours. The reaction system was first washed with water until neutral, and finally dried by adding anhydrous sodium sulfate. The solvent was recovered under re...
Embodiment 3
[0036] Add 10g (0.049mol) of dextroibuprofen and 10ml of thionyl chloride to a 50ml dry single-necked bottle, control the temperature of the oil bath at 40°C, stir and reflux until no hydrogen chloride gas is produced (about 2.5 hours), then evaporate under reduced pressure The excess thionyl chloride was removed to obtain 10.5 g (96%) of (+) α-methyl-4-(2-methylpropyl)phenylacetyl chloride as a pale yellow liquid with a strong pungent odor. Add 10g (0.045mol) of the above-mentioned liquid (+) α-methyl-4-(2-methylpropyl) phenylacetyl chloride and 5ml (0.045mol) guaiacol to a 100ml dry single-necked bottle, add 40ml of toluene was cooled in an ice-water bath and 5ml of pyridine (1.2equ) was added dropwise under strong mechanical stirring, and the raw material guaiacol basically reacted completely as detected by TLC. The reaction time is about 6 hours. The reaction system was first washed with water until neutral, and finally dried by adding anhydrous sodium sulfate. The solven...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com