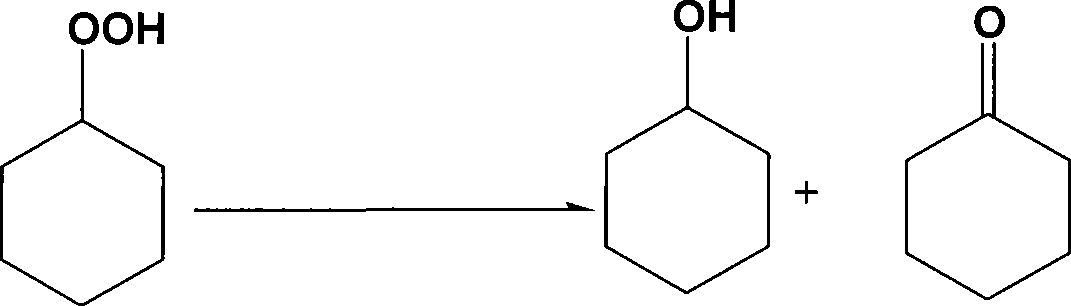
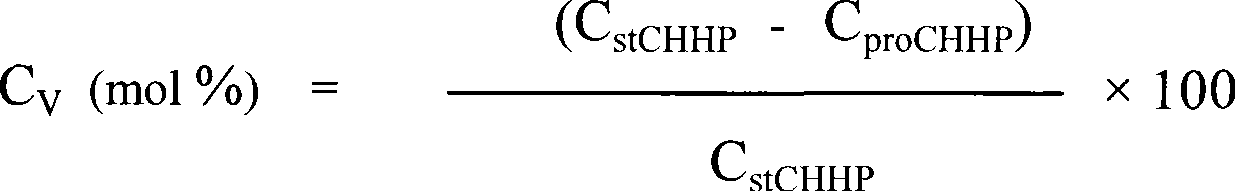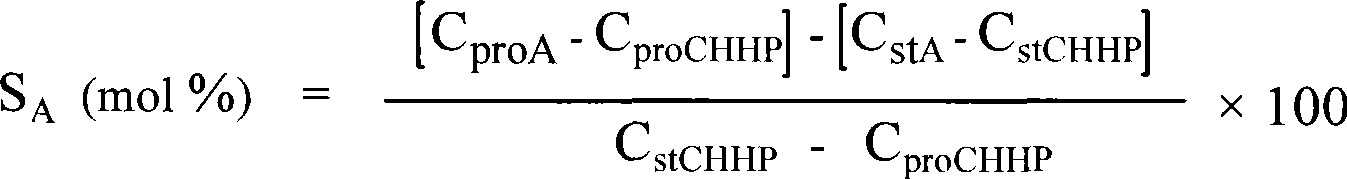Method for complex catalytic decomposition of cyclohexyl hydrogen peroxide
A cyclohexyl hydrogen peroxide, catalytic decomposition technology, applied in chemical instruments and methods, organic compounds/hydrides/coordination complex catalysts, preparation of oxygen-containing compounds, etc. High low temperature activity, good water phase stability and good overall selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Add 0.05 gram of ammonium molybdate, 0.1 gram of oxalic acid and 1 gram of water into a 50 milliliter stainless steel reaction kettle, and stir at room temperature to form a blue solution, which is an aqueous solution of complexed molybdenum oxalate. 10 g of a 2.1% cyclohexane solution containing cyclohexane hydroperoxide was added thereto. Replace the reactor with nitrogen first, then close the gas inlet and outlet valves, heat up to 85°C while stirring, continue the constant temperature reaction for 20 minutes, then cool down, and take out the upper organic phase for analysis.
[0033] The test results are shown in Table 2.
Embodiment 2
[0035] Add 0.01 gram of ammonium molybdate, 0.05 gram of tartaric acid and 1 gram of water into a 50 milliliter stainless steel reaction kettle, stir at room temperature to generate a blue solution, which is an aqueous solution of complexed molybdenum tartrate. 10 g of a 2.1% cyclohexane solution containing cyclohexane hydroperoxide was added thereto. Replace the reactor with nitrogen, then close the gas inlet and outlet valves, heat up to 125°C while stirring, continue the constant temperature reaction for 20 minutes, then cool down, and take out the upper organic phase for analysis.
Embodiment 3
[0037] Add 0.05 gram of ammonium molybdate, 0.1 gram of pyridine-2-formic acid and 1 gram of water into a 50 milliliter stainless steel reaction kettle, stir at room temperature to generate complex pyridine-2-formic acid molybdenum aqueous solution. 10 g of a 2.1% cyclohexane solution containing cyclohexane hydroperoxide was added thereto. The reaction kettle was first replaced with nitrogen, and then the gas inlet and outlet valves were tightly closed, and the temperature was raised to 105°C while stirring, and the constant temperature reaction was continued for 30 minutes, then the temperature was lowered, and the upper organic phase was taken out for analysis.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com