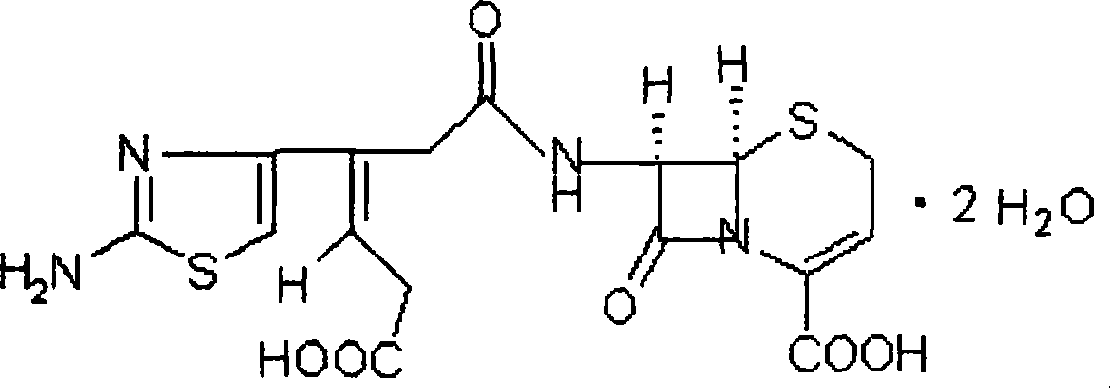
Ceftibuten sustained release tablets
A technology of ceftibuten and sustained-release tablets, which is applied in the new field of medicine, can solve problems such as inconvenience, and achieve the effects of high quality, simple method, and reduction of adverse drug reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: specification 1000 pieces
[0030] prescription:
[0031] Ceftibuten 400g
[0032] Microcrystalline Cellulose 90g
[0033] Stearic acid 80g
[0034] Ethyl cellulose 50g
[0035] Macrogol 4000 20g
[0036] Eudragit RS 100 50g
[0037] Silica 3g
[0039] Ethanol 50ml
[0040] Preparation method:
[0041] After dissolving the prescribed amount of ethyl cellulose with ethanol, add Eudragit RS 100 and Polyethylene Glycol 4000 to dissolve for later use, pass the prescribed amount of ceftibuten through a 100 mesh sieve and mix it with the prescribed amount of microcrystalline cellulose and stearic acid Evenly, add the above solution while stirring, make soft material, pass through 18 mesh sieve to granulate, dry, add prescription amount of magnesium stearate and silicon dioxide, pass through 20 mesh sieve for granulation, mix evenly, measure the content of intermediates , after passing the test, the tablet is pressed, and ...
Embodiment 2
[0042] Embodiment 2: specification 10000 pieces
[0043] prescription:
[0044] Ceftibuten 4000g
[0045] Microcrystalline Cellulose 900g
[0046] Stearic acid 800g
[0047] Ethyl cellulose 500g
[0048] Macrogol 4000 200g
[0049] Eudragit RS 100 500g
[0050] Silica 30g
[0052] Ethanol 550ml
[0053] Preparation method:
[0054] After dissolving the prescribed amount of ethyl cellulose with ethanol, add Eudragit RS 100 and Polyethylene Glycol 4000 to dissolve for later use, pass the prescribed amount of ceftibuten through a 100 mesh sieve and mix it with the prescribed amount of microcrystalline cellulose and stearic acid Evenly, add the above solution while stirring, make soft material, pass through 18 mesh sieve to granulate, dry, add prescription amount of magnesium stearate and silicon dioxide, pass through 20 mesh sieve for granulation, mix evenly, measure the content of intermediates , after passing the test, the tablet is pre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com