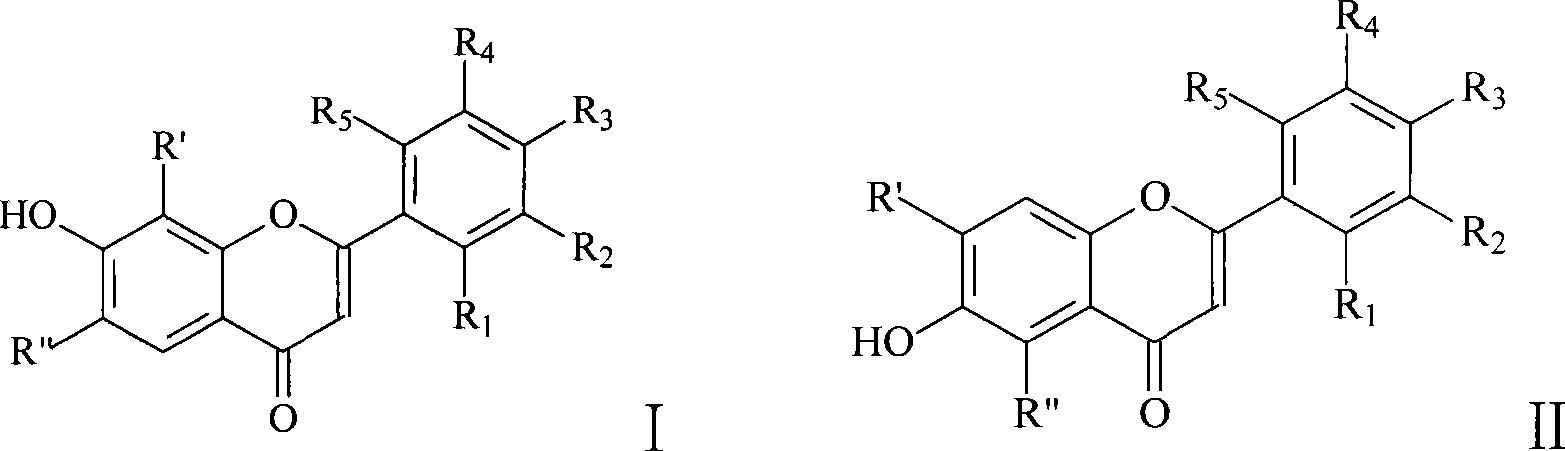
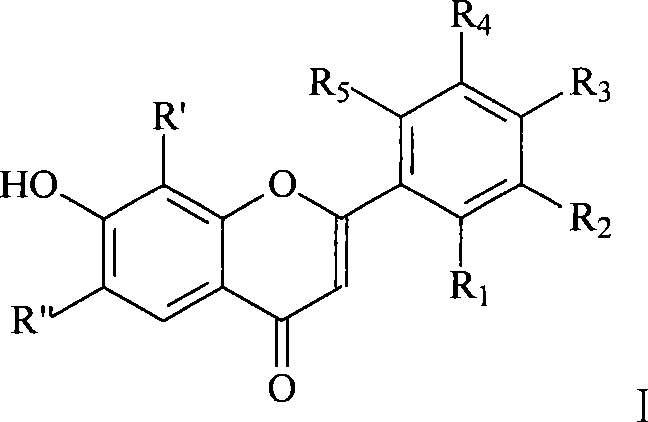
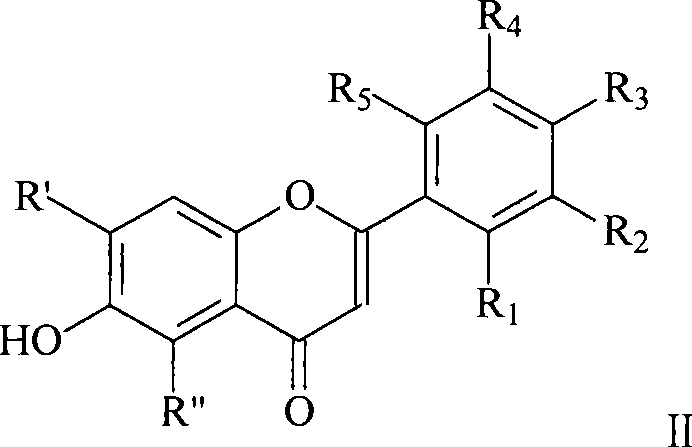
Flavonol derivative and use for anti-platelet aggregation
An anti-platelet aggregation and hydroxyflavone technology, applied in the field of medicine, can solve the problems of toxic and side effects, inaccurate curative effect, insufficient stability and the like, and achieve the effect of a simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Put 0.03mol 2',4'-dihydroxyacetophenone, 0.22mol anhydrous potassium carbonate, and 150mL dry acetone into a 250mL round bottom flask, and slowly add 0.06mol o-fluorobenzoyl chloride dropwise at room temperature. After dropping, it was heated to reflux for 12 hours. Cool, filter with suction, and wash the filter cake with a small amount of acetone. The filter cake was acidified to pH 4-5 with dilute hydrochloric acid, suction filtered, washed and dried. Recrystallization was done with acetone. Add 0.022mol of 1-[2-hydroxyl-4-(2-fluorobenzoyloxy)phenyl]-3-(2-fluorophenyl)]-1,3-propanedione to 100mL cone after recrystallization Add 36mL of concentrated sulfuric acid to a flask, place in an ice bath, and stir for 4 hours. The reaction was stopped, and the reaction solution was poured into a large amount of ice water, and a pale white solid was precipitated. Suction filtration, then add the filter cake into 150mL 5% potassium carbonate solution, boil and cool, the preci...
Embodiment 2
[0020] According to the method for embodiment 1, by 2 ', 4'-dihydroxyacetophenone, o-toluyl chloride reaction obtains white solid. Yield: 38.4%. MS m / z (M): 252.26. 1 H-NMR (DMSO), δ (ppm): 2.54 (3H, s), 6.62 (1H, s), 6.89 (1H, s), 7.44 (2H, m), 7.67 (2H, m), 7.92 (2H , m), 10.92 (1H, s).
Embodiment 3
[0021] Example 3: 2'-chloro-7-hydroxyflavone
[0022] According to the method for embodiment 1, by 2', 4'-dihydroxyacetophenone, o-chlorobenzoyl chloride reaction obtains white solid. Yield: 27.6%. MS m / z (M): 272.68. 1 H-NMR (DMSO), δ (ppm): 6.49 (1H, s), 6.88 (1H, s), 6.89-6.98 (1H, m), 7.51-7.69 (4H, m), 7.76-7.79 (1H, d), 10.94 (1H, s).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com