Anti-tumor pharmaceutical composition
A technology of composition and medicine, applied in the field of pharmacy, capable of solving problems such as allergic reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] Extraction of apophyllic alkaloids from Corydalis
[0100] (1) Soaking and filtering
[0101] The rhizome of Corydalis yanhusuo was 5.6Kg. After being completely crushed by a pulverizer, it was refluxed with a volume of 30L ethanol (concentration 95%) for 3 hours, and then filtered to remove the medicinal powder residue.
[0103] Distill and recover 30L ethanol, the temperature does not exceed 60 ℃, reflux for 2 hours, remove the extract in the reactor to the collection bucket (the extract is brownish-red, slightly viscous).
[0104] (3) The second heat reflow
[0105] Use the recovered 30L ethanol for the second heat reflux, and the filtration and distillation requirements are the same as before.
[0106] (4) The third heat reflow
[0107] Use the recovered 30L ethanol for the third heat reflux, and the filtration and distillation requirements are the same as before. 355 g of total extract was obtained.
[0108] (5) Extraction of total alkaloi...
Embodiment 2
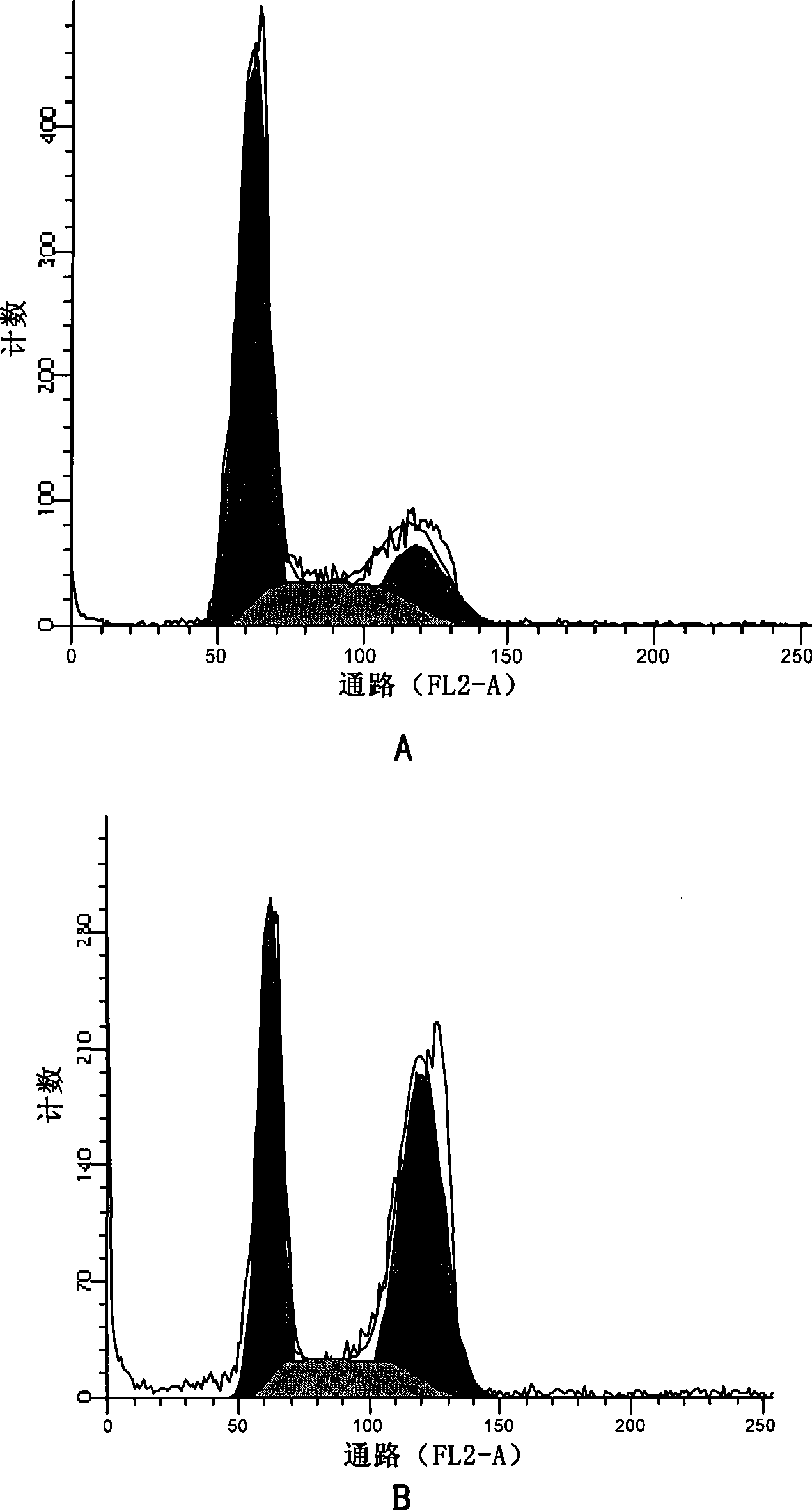
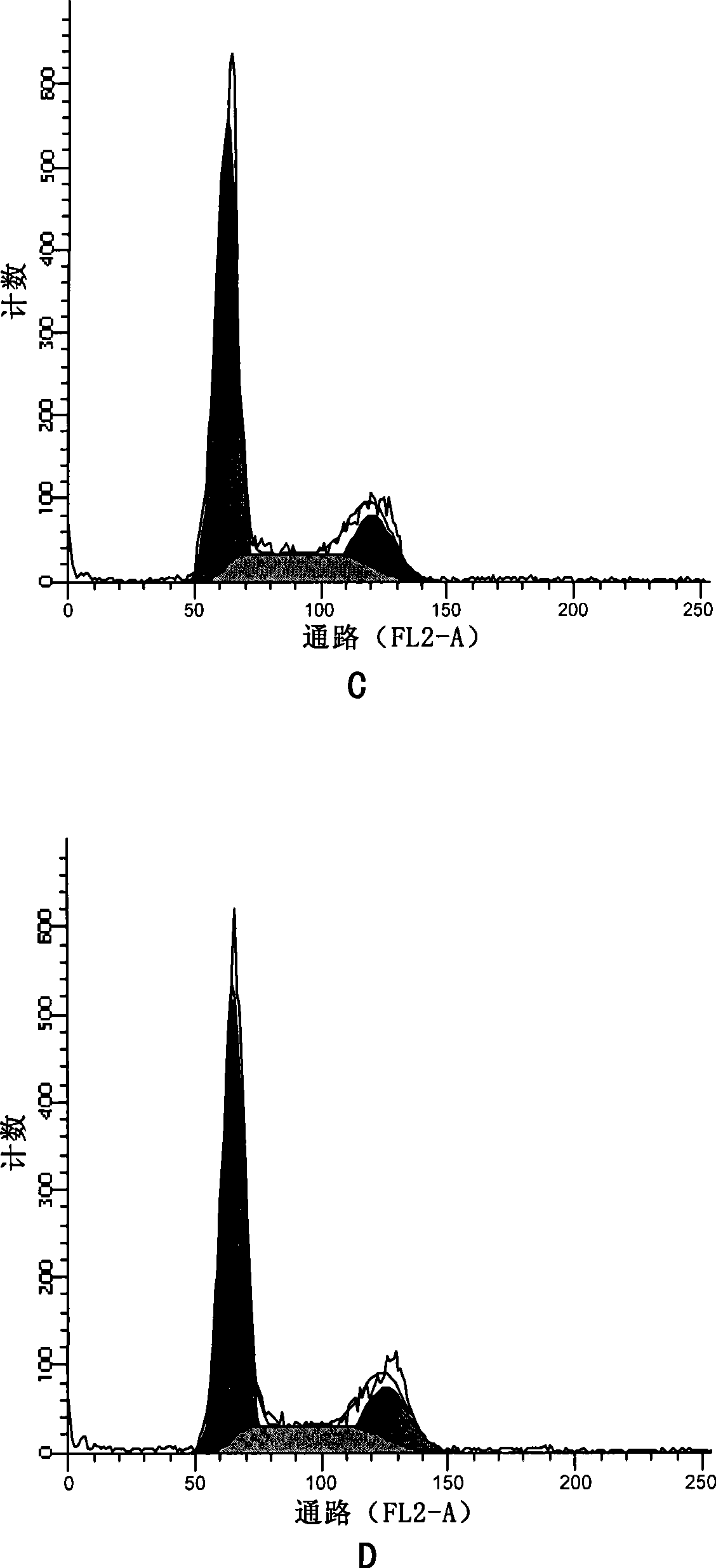
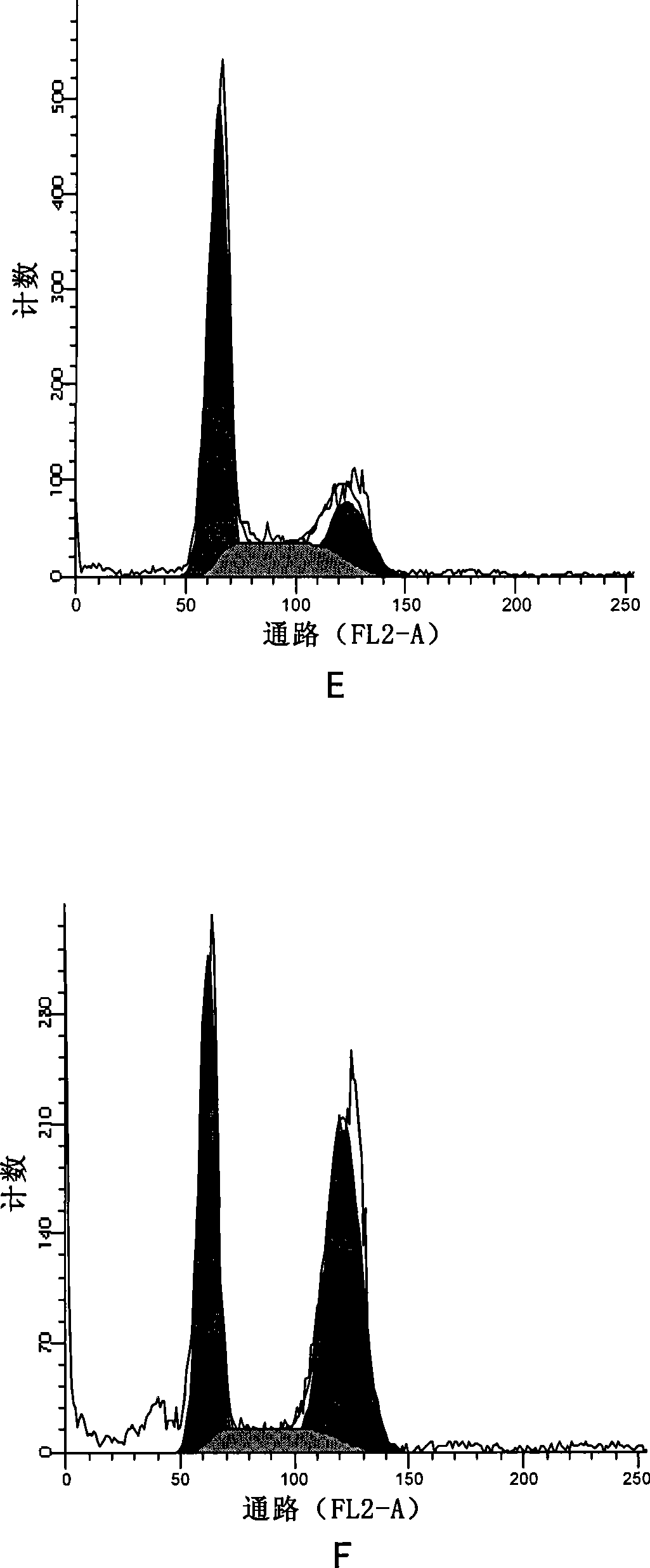
[0123] Effects of apophyllic alkaloids on the cell cycle of human gastric cancer HGC
[0124] Experimental drugs:
[0125] Sea papaverine (a compound represented by formula II) was prepared in Example 1, paclitaxel was purchased from Sichuan Jiufeng Natural Pharmaceutical Co., Ltd., and vincristine was purchased from Shanghai Santikangsheng Phytochemical Co., Ltd.
[0126] Culture HGC cells in vitro. When the growth status is good, digest and inoculate 1×10 5 Cells adhered to the wall overnight in a 6cm petri dish. After the cells are in good condition, aspirate the medium and divide them into 7 groups randomly:
[0127] For group A alone, add sea papaverine diluted in culture medium to a final concentration of 2μM;
[0128] In group b alone, add sea papaverine diluted with culture medium to a final concentration of 50 nM;
[0129] Group c was treated with medicine alone, and vincristine diluted in culture medium was added to the final concentration of 10 nM;
[0130] In group d w...
Embodiment 3
[0138] Inhibitory effect of apophyl alkaloids and mitotic inhibitors on tumor growth in S180 tumor-bearing mice
[0139] Experimental animals:
[0140] Male SPF grade KM mice, purchased from Shanghai Slack Laboratory Animal Co., Ltd., weighing 18-22 g, raised in an SPF grade animal room, 12 hours of light / 12 hours of darkness, and free intake of feed and water.
[0141] Experimental drugs:
[0142] Sea papaverine, prepared in Example 1, paclitaxel was purchased from Sichuan Jiufeng Natural Pharmaceutical Co., Ltd., apomorphine (shown in structural formula V) was purchased from Qinghai Pharmaceutical Factory Co., Ltd., corydine (shown in structural formula IV) ) Was purchased from Hunan Neuer Biopharmaceutical Co., Ltd., and kaempferine (shown in structural formula III) was purchased from NADUCS, Inc., USA.
[0143] experimental method:
[0144] Except for the normal group, take 7-day-inoculated mice with S180 ascites, extract the ascites under aseptic conditions, and adjust the ce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com