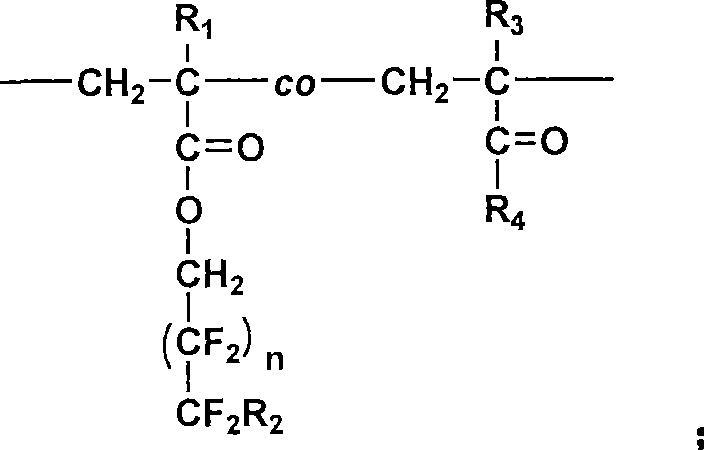
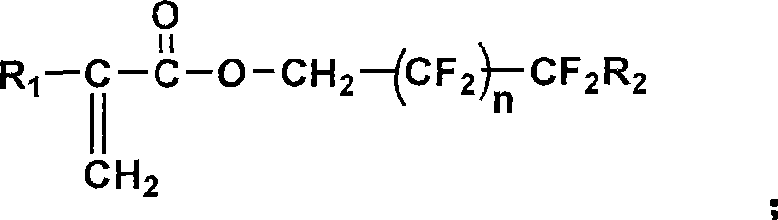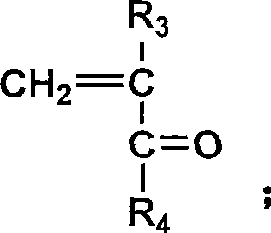Random copolymerization fluorine-containing macromole emulsifying agent and preparation thereof
A macromolecular emulsifier and molecular weight technology, which is applied in the field of fluorine-containing macromolecular emulsifiers, can solve the problems of impure products, low polymerization reaction conditions and equipment requirements, and difficult industrial control and production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Example 1: Preparation of Poly(DFHMA-co-DMAEMA)
[0089] (1) Preparation of reaction monomers and initiators: methacrylic acid-2-(dimethylamino)ethyl ester and methacrylic acid-(2,2,3,3,4,4,5,5,6,6 , 7,7-dodecafluoro) heptyl ester (DFHMA) was used with activated basic Al 2 o 3 Column treatment, and then vacuum distillation for later use; AIBN recrystallized with absolute ethanol for later use.
[0090](2) Preparation of polymer: take hydrophobic monomer methacrylate-(2,2,3,3,4,4,5,5,6,6,7,7-dodecafluoro)heptyl ( DFHMA) 4.41g (0.011mol), hydrophilic monomer methacrylic acid-2-(dimethylamino) ethyl ester (DMAEMA) 15.59g (0.099mol); 0.22g initiator AIBN (according to the total monomer molar weight 1.2% by weighing); Measure 1mL of n-dodecanethiol, dissolve it in 101mL of dehydrated ethanol (measured according to the monomer solid content of 20%), after stirring evenly, pour into In a 250mL four-neck bottle with a thermometer and a nitrogen conduit, pass N 2 , stirring...
Embodiment 2
[0095] Example 2: Preparation of Poly(DFHMA-co-DMAEMA)
[0096] (1) Preparation of reaction monomers and initiators: same as step [1] of embodiment one.
[0097] (2) Preparation of polymer: take hydrophobic monomer methacrylate-(2,2,3,3,4,4,5,5,6,6,7,7-dodecafluoro)heptyl ( DFHMA) 7.78g (0.019mol); Hydrophilic monomer methacrylate-2-(dimethylamino) ethyl ester (DMAEMA) 12.22g (0.078mol); 0.19g initiator AIBN (according to the total monomer molar weight 1.2% by weighing); Measure 1mL of n-dodecanethiol, dissolve it in 101mL of dehydrated ethanol (measured according to the monomer solid content of 20%), after stirring evenly, pour into In a 250mL four-neck bottle with a thermometer and a nitrogen conduit, pass N 2 , stirring at a stirring speed of 350r / min, rapidly raising the temperature to 70°C, and maintaining this temperature and stirring speed, reacting for 7h, cooling and discharging.
[0098] (3) Purification of polymer: carry out by the method for embodiment one (3). ...
Embodiment 3
[0101] Example Three: Preparation of Poly(DFHMA-co-DMAEMA)
[0102] (1) Preparation of reaction monomer and initiator: carry out by the method of embodiment one (1).
[0103] (2) Preparation of polymer: Weigh strong hydrophobic monomer methacrylate-(2,2,3,3,4,4,5,5,6,6,7,7-dodecafluoro)heptyl (DFHMA) 10.44g (0.026mol); Hydrophilic monomer methacrylic acid-2-(dimethylamino) ethyl ester (DMAEMA) 9.56g (0.061mol); 0.17g initiator AIBN (by total monomer molar weight 1.2% of 1.2% by weighing); Measure 1mL of n-dodecanethiol, dissolve it in 101mL (according to 20% solid content) of dehydrated ethanol, after stirring evenly, pour into In the 250mL four-neck bottle with nitrogen conduit, pass N 2 , stirring at a stirring speed of 350r / min, rapidly raising the temperature to 70°C, and maintaining this temperature and stirring speed, reacting for 7h, cooling and discharging.
[0104] (3) Purification of polymer: carry out by the method for embodiment one (3).
[0105] (4) Characteri...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com