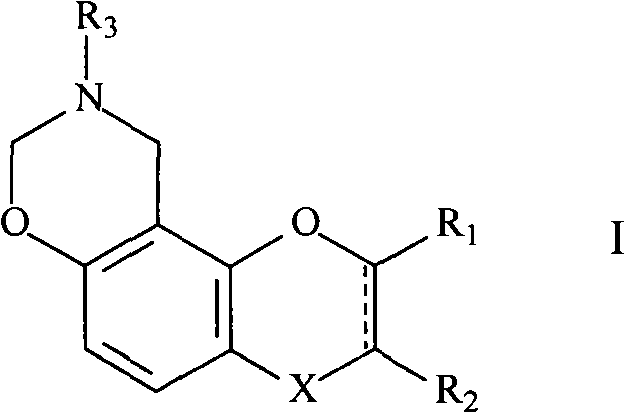
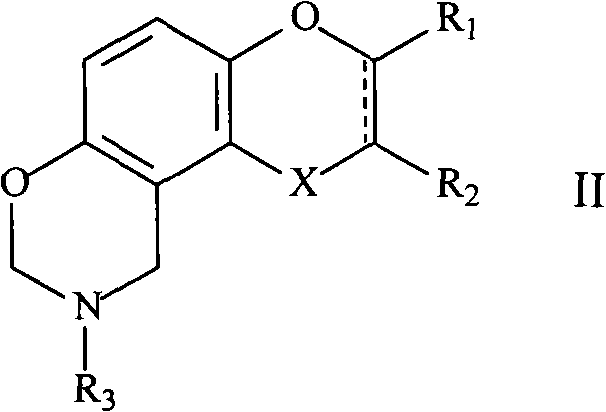
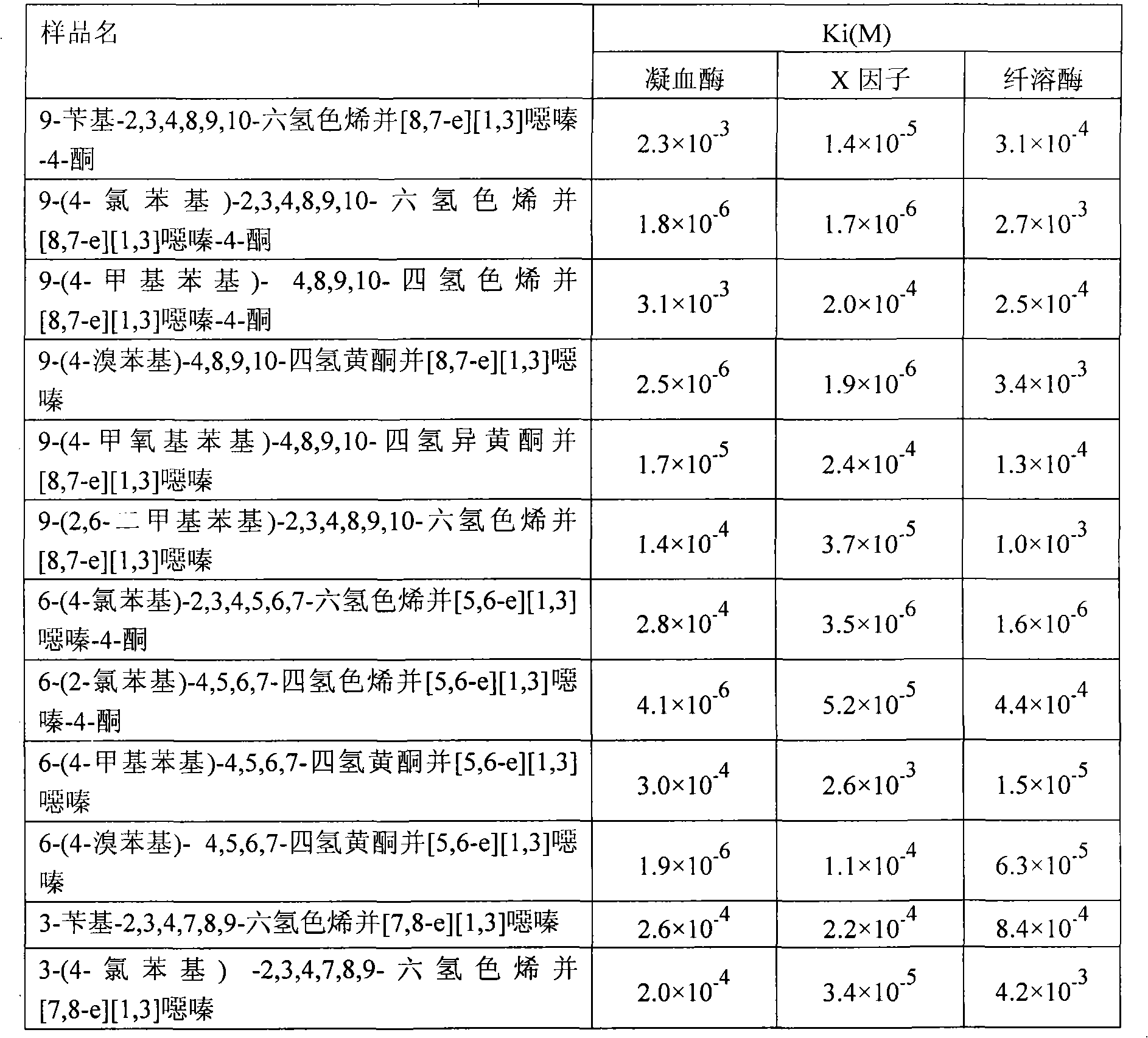
Novel oxazine compound and use for preventing platelet aggregation
A compound and oxazine technology, applied in the field of medicine, can solve the problems of poor stability and low content of natural flavonoids, and achieve the effect of a simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: 9-phenyl-4,8,9,10-tetrahydroflavone[8,7-e][1,3]oxazine
[0024] Mix 1.2mL 37% formaldehyde aqueous solution with 5mmol aniline dissolved in 2-3mL 95% ethanol, heat to reflux and stir for a few minutes, then add 5mmol 7-hydroxyflavone dissolved in 10mL 95% ethanol, heat to reflux and stir overnight. After cooling, a large amount of white precipitate was precipitated, which was filtered by suction, and finally recrystallized by ethanol to obtain white crystals with a yield of 44%. MS m / z (M): 355.39. 1 HNMR (CDCl 3 ): δ4.61 (2H, s), 6.00 (2H, s), 6.40 (1H, d), 6.60 (3H, m), 6.71 (1H, s), 7.08~7.34 (8H, m). δ
Embodiment 2
[0025] Example 2: 9-Benzyl-4,8,9,10-tetrahydroflavone[8,7-e][1,3]oxazine
[0026] According to the preparation method provided in Example 1, the compound is obtained by reacting 7-hydroxy-flavone with aqueous formaldehyde and benzylamine. Yield: 47%. MS m / z (M): 369.41. 1 HNMR (CDCl 3 ): δ3.62 (4H, s), 5.01 (2H, s), 6.40 (1H, d), 6.60 (3H, m), 6.71 (1H, s), 7.06-7.21 (8H, m).
Embodiment 3
[0027] Example 3: 9-(2-methylphenyl)-4,8,9,10-tetrahydroflavone[8,7-e][1,3]oxazine
[0028] According to the preparation method provided in Example 1, the compound is obtained by reacting 7-hydroxy-flavone with aqueous formaldehyde and 2-methylaniline. Yield: 38%. MS m / z (M): 369.41. 1 HNMR (CDCl 3 ): δ2.35(3H, s), 4.61(2H, s), 6.00(2H, s), 6.40(1H, d), 6.47(2H, m), 6.71(1H, s), 6.89(2H, m), 7.14-7.34 (6H, m).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com