Regulation of protein activity by reversible acetylation
An acetylation and activity technology, applied in the field of mitochondrial matrix proteins, can solve the unstudied problems such as the precise function and regulation of AceCS2
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0234] Preparation and screening of combinatorial chemical libraries is well known to those skilled in the art. Such combinatorial chemical libraries include, but are not limited to, peptide libraries (see, eg, US Patent No. 5,010,175; Furka, 1991, Int J PeptProt Res 37:487-493 (1991) and Houghton et al., 1991, Nature 354:84-88). Other chemistries can also be utilized to generate chemical diversity libraries. These chemicals include, but are not limited to: peptidomimetics (eg, PCT Publication No. WO 91 / 19735), encoded peptides (eg, PCT Publication No. WO 93 / 20242), random biooligomers (eg, PCT Publication No. WO 92 / 00091 ), benzodiazepines Classes (e.g., U.S. Pat. No. 5,288,514), diversomers such as hydantoins, benzodiazepines and dipeptides (Hobbs et al., 1993, Proc Natl Acad Sci USA90:6909-6913), alkene polypeptides (Hagihara et al., 1992, J Amer Chem Soc 114:6568), non-peptidic peptide mimetics containing glucose scaffolds ( Hirschmann et al., 1992, J Amer Chem Soc 11...
Embodiment 1
[0385] Example 1: general method
[0386] A. Cell culture and transfection
[0387] 37°C, 5% CO 2 HEK293, COS-1 and HeLa cells were cultured and grown in DMEM supplemented with 10% FCS, 2 mM L-glutamine, 100 U / mL penicillin and 100 μg / mL streptomycin. Calcium phosphate transfection was used to transfect HEK293 cells (Chen and Okayama, 1987, MolCell Biol 7:2745-52). Hela and Cos-1 cells were transfected with Fugene from Roche. Stable expression of AceCS2 was generated by selection in complete DME growth medium containing 800 μg / mL Geneticin (Invitrogen). 标志 , SIRT3 标志 、SIRT3-H248Y 标志 or contain empty 标志 - Control vector pcDNA 标志 HEK293 cell line.
[0388] B. Plasmids and mutagenesis
[0389] All expression constructs were generated using standard PCR-based cloning protocols and verified by DNA sequencing. PCR primer 5′-CG GAATTC CATGGCGGCGCGCACCCTGGGC-3' (SEQ ID NO: 15) and 5'-CG GAATTC CTTAGCAGCAGCCTGCTTGTCCTTGC-3' (SEQ ID NO: 16) (each containing an EcoRI rest...
Embodiment 2
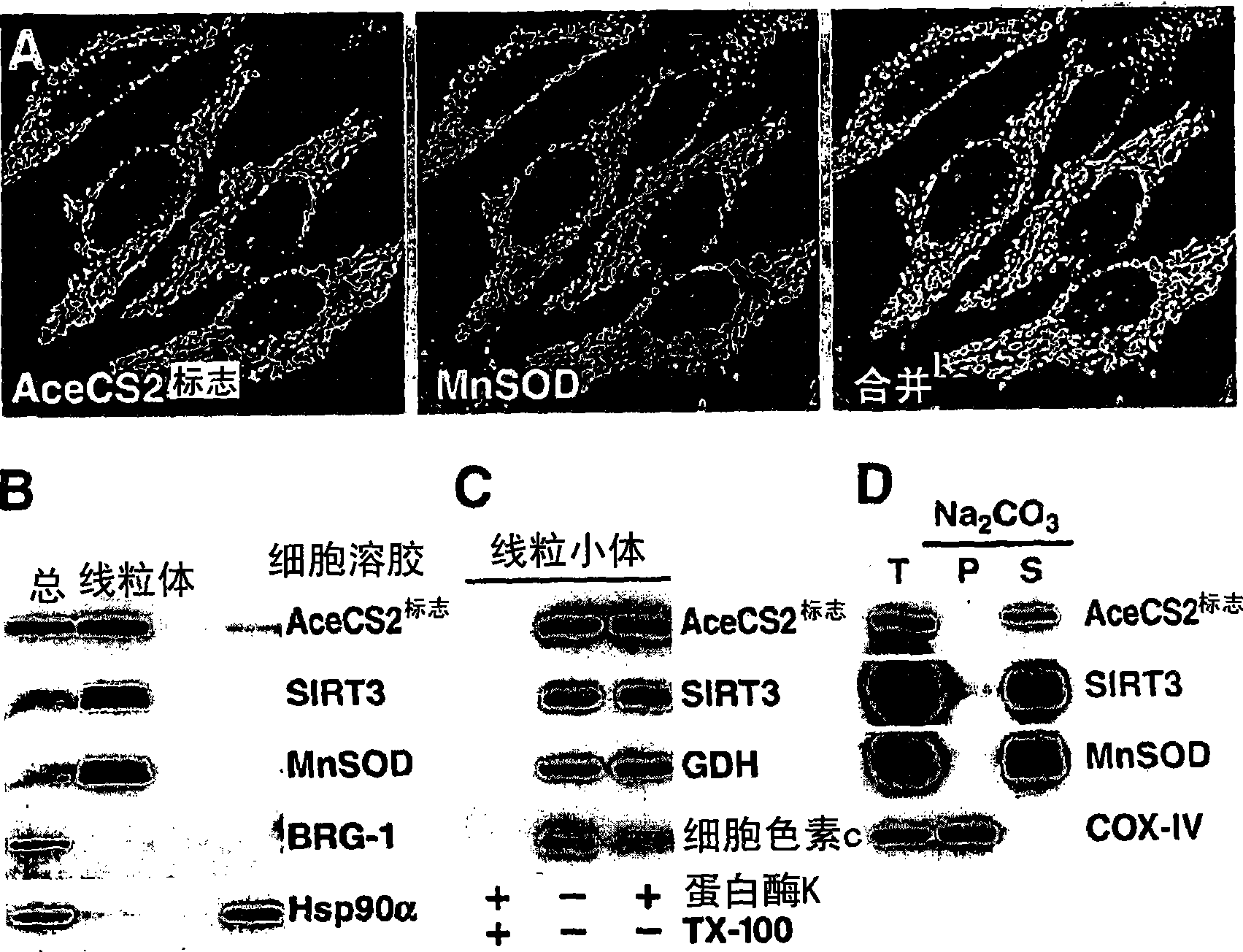
[0424] Example 2: Identification of acetyl-CoA synthetase (AceCS2) as a cellular target of SIRT3
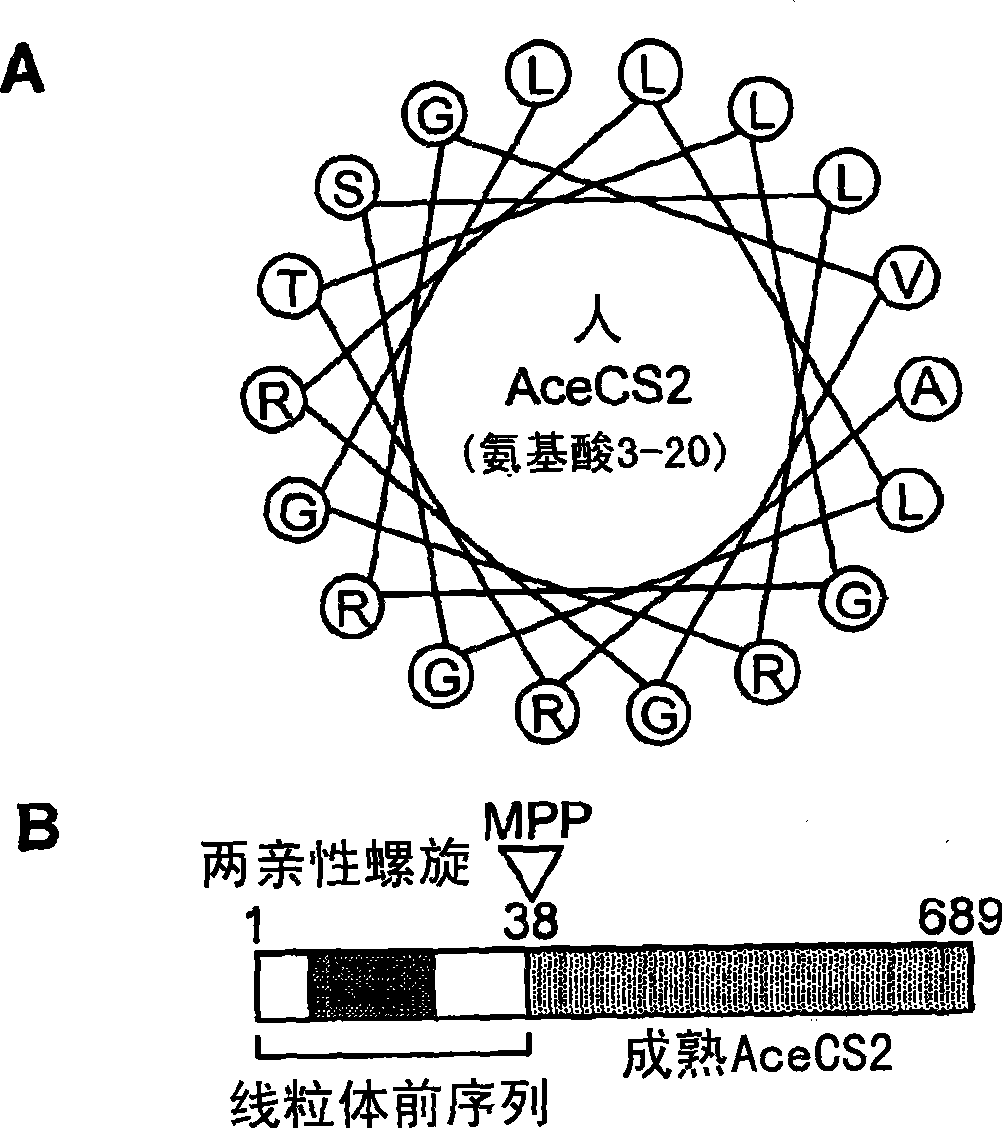
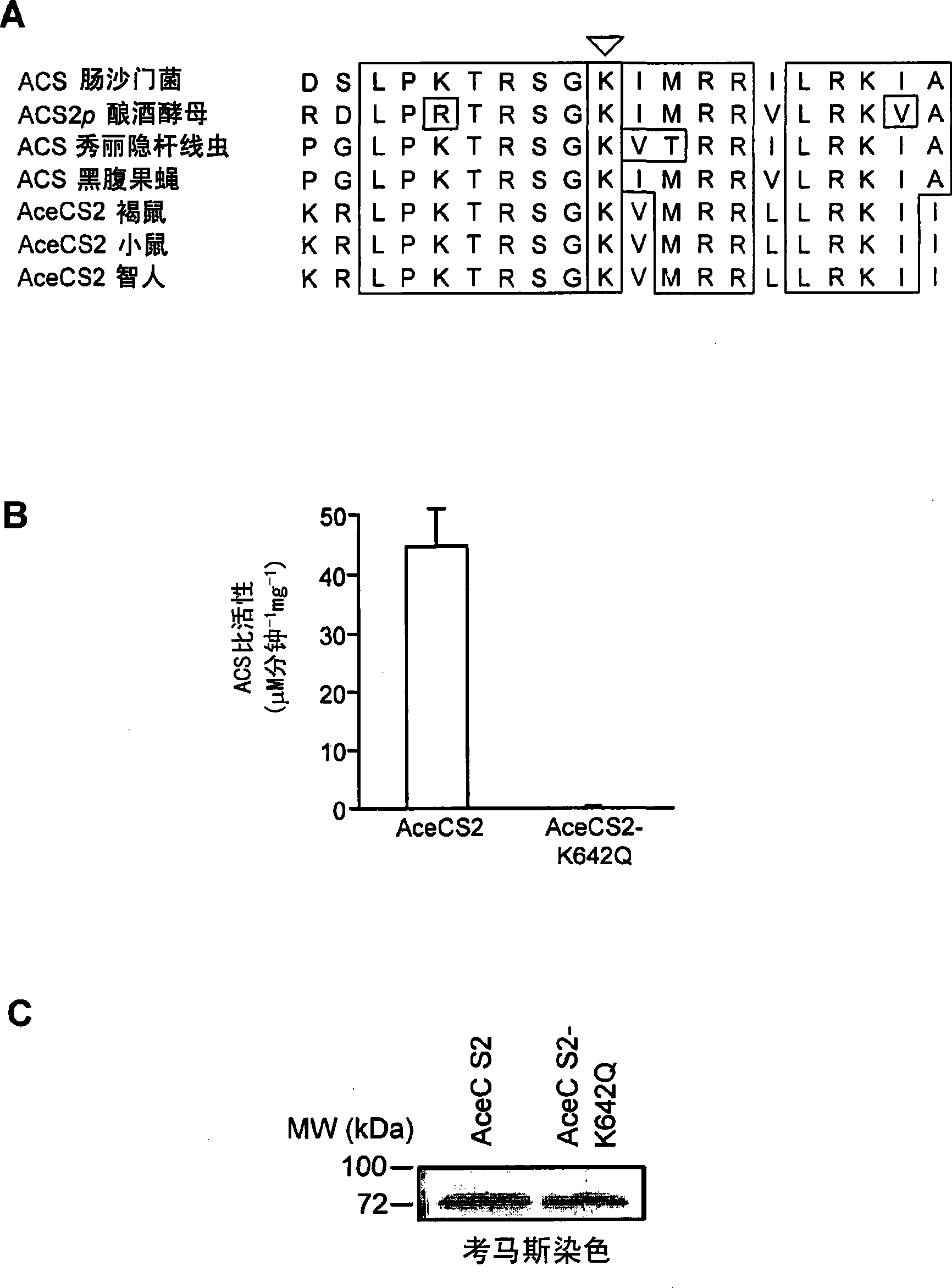
[0425] As a protocol to identify lysine acetylated mitochondrial proteins targeted by SIRT3, 4, or 5, the sequence was studied in relation to the acetylated lysine residues found in Salmonella enterica acetyl-CoA synthetase (ACS). Human proteins with similar domain sequences. The acetyl-lysine-containing region of Salmonella enterica ACS was chosen because it is a known substrate of the Sir2-like protein CobB (Starai et al., 2003, Genetics 163:545-55; Starai et al., 2002, Science 298:2390- 2). In the second step, the mitochondrial localization probabilities of human proteins showing high similarity were analyzed using MITOPROT II and PREDOTAR version 1.03 subcellular prediction software (Small et al., 2004, Proteomics 4:1581-90; Claros and Vincens, 1996, Eur J Biochem 241:779-86). This study obtained a human acetyl-CoA synthetase protein with a high probability of localizati...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com