Anti-hepatitis b virus medicine dispersion combination at non-crystal state and medicine preparation thereof
A pharmaceutical preparation and composition technology, applied in the field of non-crystalline anti-hepatitis B virus drug dispersion composition and pharmaceutical preparations, can solve the problem of affecting the crystal form and chemical stability of adefovir dipivoxil, affecting chemical stability, etc. problem, achieve the effect of enhancing chemical stability and crystal form stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Example 1 Preparation of adefovir dipivoxil without crystalline form and entecavir without crystalline form and their stability
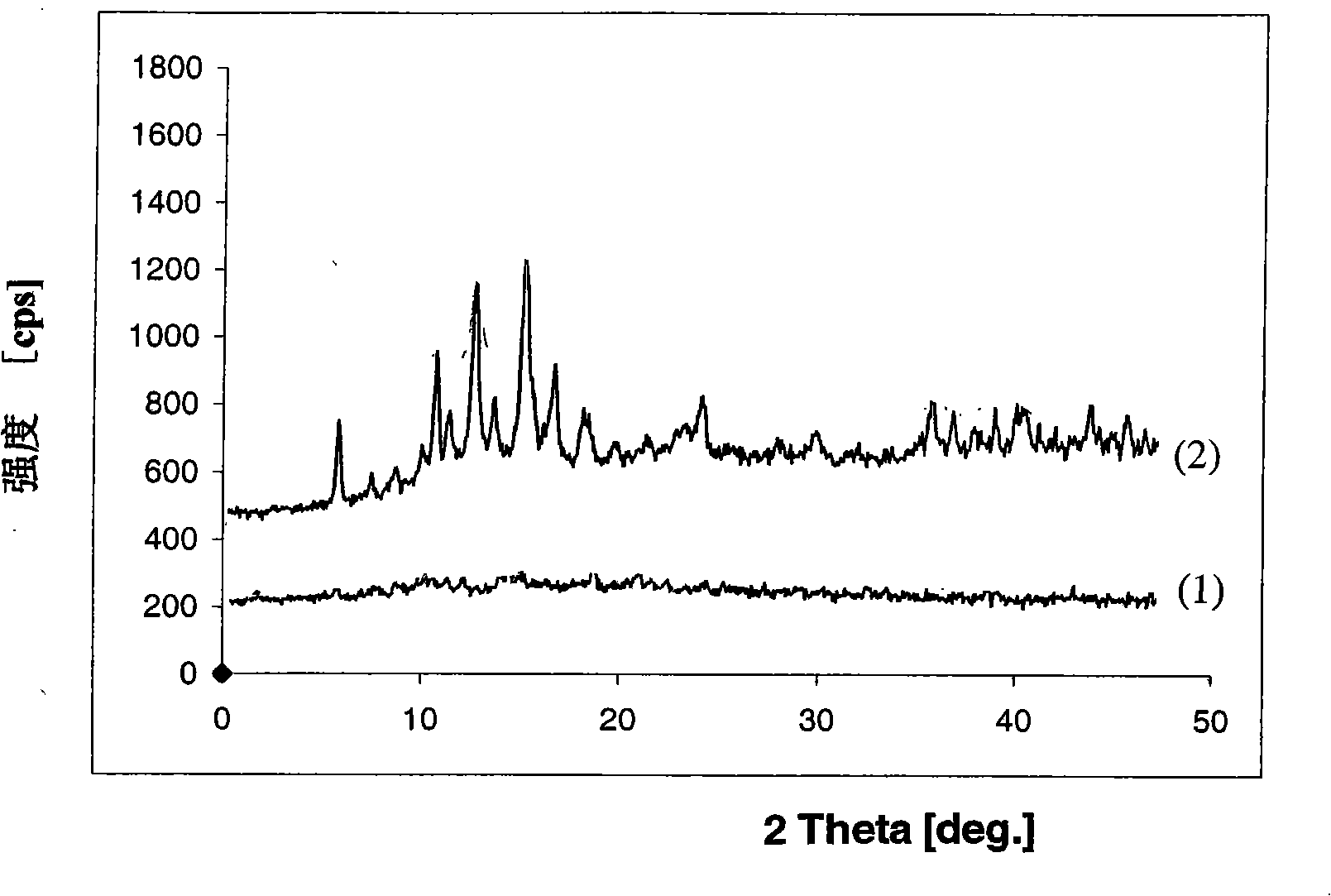
[0072] Method A: Dissolve 5.0 g of adefovir dipivoxil in crystalline form in 250 ml of ethanol solvent heated to 40-50 degrees (Celsius, the same below). After the resulting clear solution is cooled to room temperature (25-30 degrees), use a small spray dryer (Buchi Model-190 type) and nitrogen to spray dry all the solutions, and control the inlet temperature to 119-130 degrees and the outlet temperature to 50-65 degrees , The white oil thus obtained is adefovir dipivoxil (4.3 g) in the non-crystalline state. The adefovir dipivoxil of the obtained non-crystalline state is packed in 75 milliliters of plastic bottles (HDFE), and seals, and is placed in 40 degree / 75% relative humidity state and keeps three months, can observe with naked eyes 55-10% of the crystalline material is distributed in the oily form of adefovir dipivoxil. The results o...
Embodiment 2
[0074] Example 2 Preparation of the dispersion composition of adefovir dipivoxil without crystalline form and polyvinylpyrrolidone-ethylene acetate without crystalline form
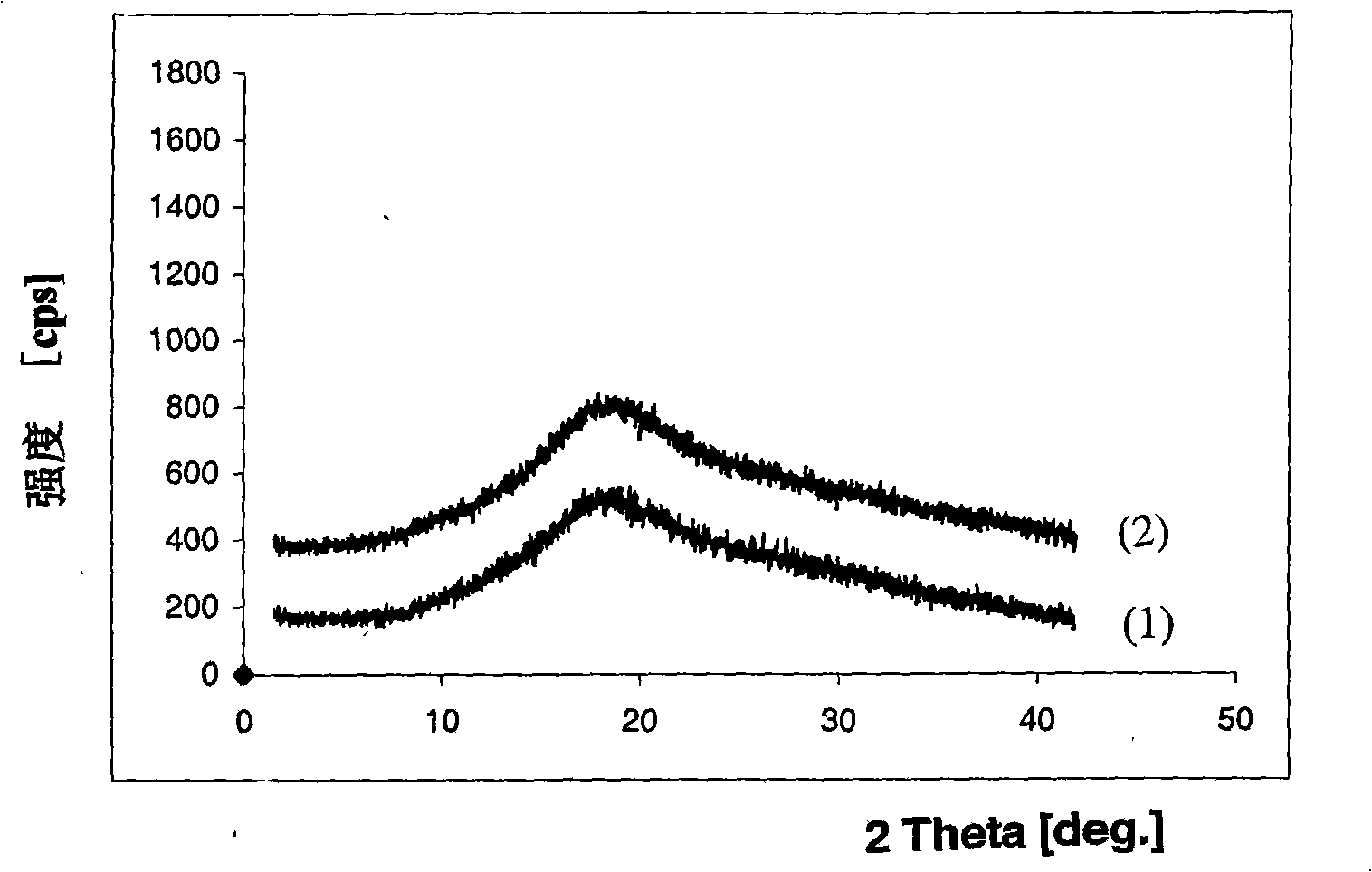
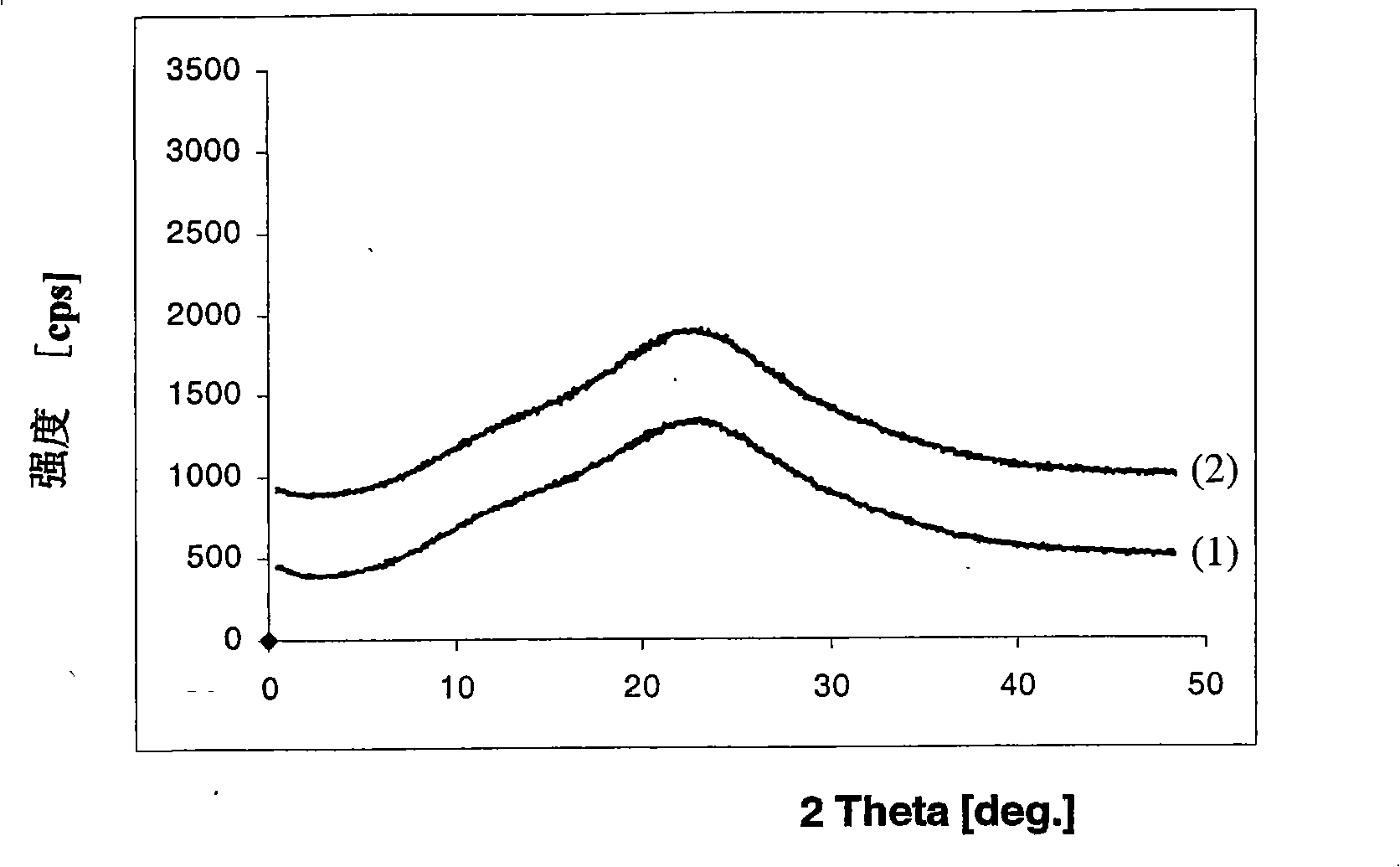
[0075] Method C: Dissolve 5.0 g of adefovir dipivoxil in crystalline form and 10.0 g of polyvinylpyrrolidone-ethylene acetate (PVP-VA64, PlasdoneS-630, copovidone, the same below) without crystalline form in 250 ml, Heat the ethanol solvent to 50 degrees and keep stirring the solution. The hot solution thus obtained was further heated to boiling, and all solvent was evaporated by rotary distillation under reduced pressure (30-80 mmHg) to obtain a solid. The obtained solid was dried under vacuum at 40°C for 15 hours. Grinding the dried solid, further drying at a temperature between 35°C and 40°C for about 12 hours to remove the solvent, to obtain a dispersion composition of adefovir dipivoxil and polyvinylpyrrolidone-ethylene acetate without crystals (13.2g). X-ray powder diffraction pattern (attached fi...
Embodiment 3
[0078] Example 3 Preparation of the dispersion composition of entecavir without crystalline form and polyvinylpyrrolidone-ethylene acetate without crystalline form
[0079] Method F: Dissolve 5.0 g of crystalline entecavir and 10.0 g of non-crystalline polyvinylpyrrolidone-vinyl acetate in 200 ml of ethanol solvent heated to 50 degrees, and keep stirring the solution. The hot solution thus obtained was further heated to boiling, and all solvent was evaporated by rotary distillation under reduced pressure (30-80 mmHg) to obtain a solid. The obtained solid was dried under vacuum at 40°C for 15 hours. Grind the dried solid, and then further dry at a temperature between 35°C and 40°C for about 12 hours to remove the solvent to obtain a dispersion composition (13.0g) of entecavir and polyvinylpyrrolidone-ethylene acetate without crystals . X-ray powder diffraction pattern (see attached figure 2 -(1)) proves that the obtained product belongs to the non-crystalline form of enteca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com