'Relingqing' chewable tablet and preparation method thereof
A chewable tablet, hot drenching technology, applied in the field of medicine, can solve the problems of personal privacy impact, decreased stability, increased hygroscopicity, etc., and achieves the effects of high bioavailability, convenient administration, and fast release speed.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] 1. Prescription
[0064]
[0065] 2. Preparation method:
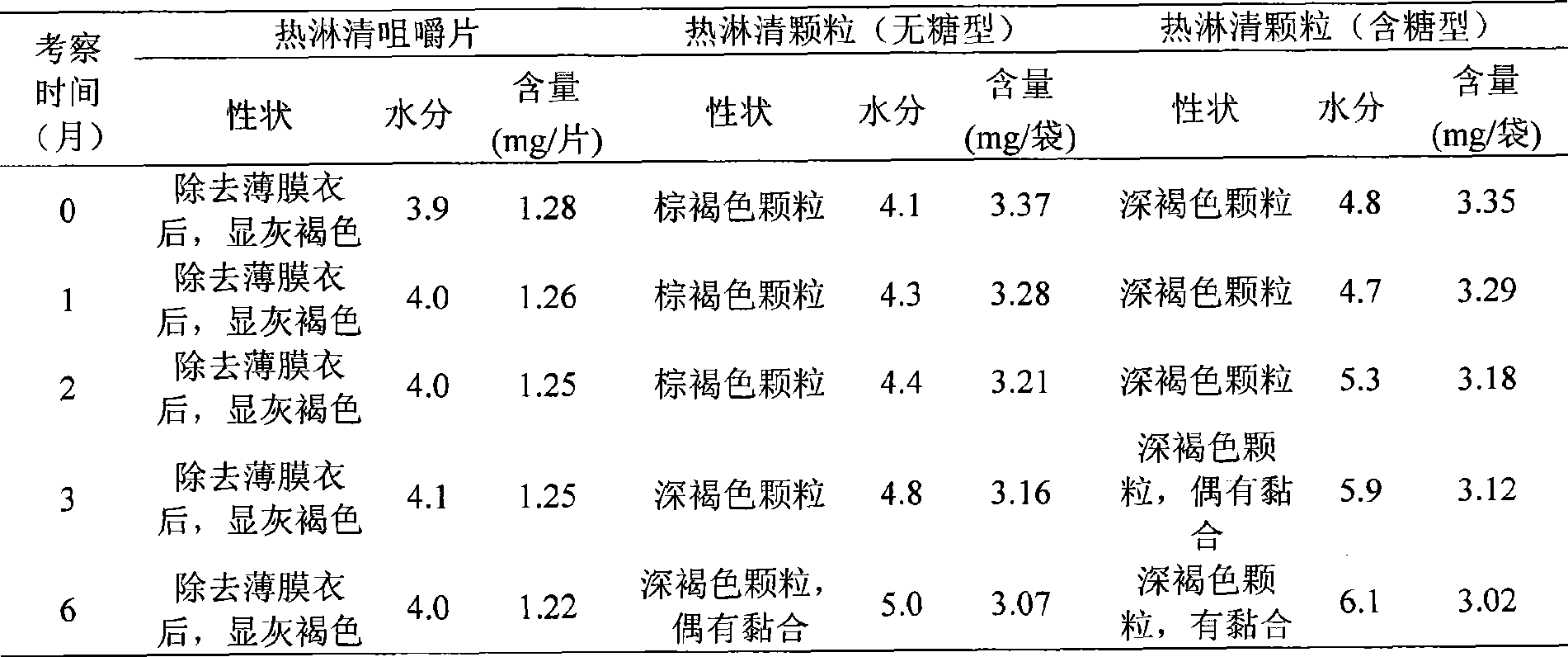
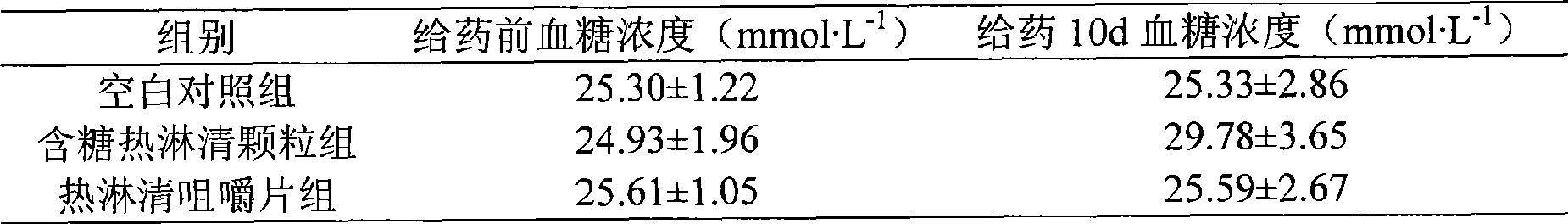
[0066] Take 3300g of Polygonum japonica medicinal materials, add water to decoct twice for 1.5 hours each time, combine the decoctions, filter, and concentrate the filtrate under reduced pressure to a thick paste with a relative density of 1.35-1.38 (60°C), vacuum dry, and pulverize into fine Powder, add mannitol 180g, sorbitol 180g, steviol 5g, citric acid 10g, mix well, granulate with 80% ethanol, dry at low temperature (60°C), add magnesium stearate, mix well, and press into 0.7g The finished tablet of the present invention can be obtained as the finished product of the hot shower clear chewable tablet, and the tablet can also be coated with a film coating as required. Measured by HPLC, each Relinqing chewable tablet contains Polygonum capsulata to quercetin (C 15 H 10 O 7 ·H 2 O), it is 1.12 mg.
Embodiment 2
[0068] 1. Prescription
[0069]
[0070] 2. Preparation method: refer to Example 1. Measured by HPLC, each Relinqing chewable tablet contains Polygonum capsulata to quercetin (C 15 H 10 O 7 ·H 2 O), it is 1.03mg.
Embodiment 3
[0072] 1. Prescription
[0073]
[0074] 2. Preparation method: refer to Example 1. Measured by HPLC, each Relinqing chewable tablet contains Polygonum capsulata to quercetin (C 15 H 10 O 7 ·H 2 O), it is 1.27mg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com