Particles for delivery of active ingredients, process of making and compositions thereof
A technology of active ingredients and compositions, applied in the field of active ingredient delivery, which can solve the problems of undescribed active ingredient delivery and achieve the effect of high surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
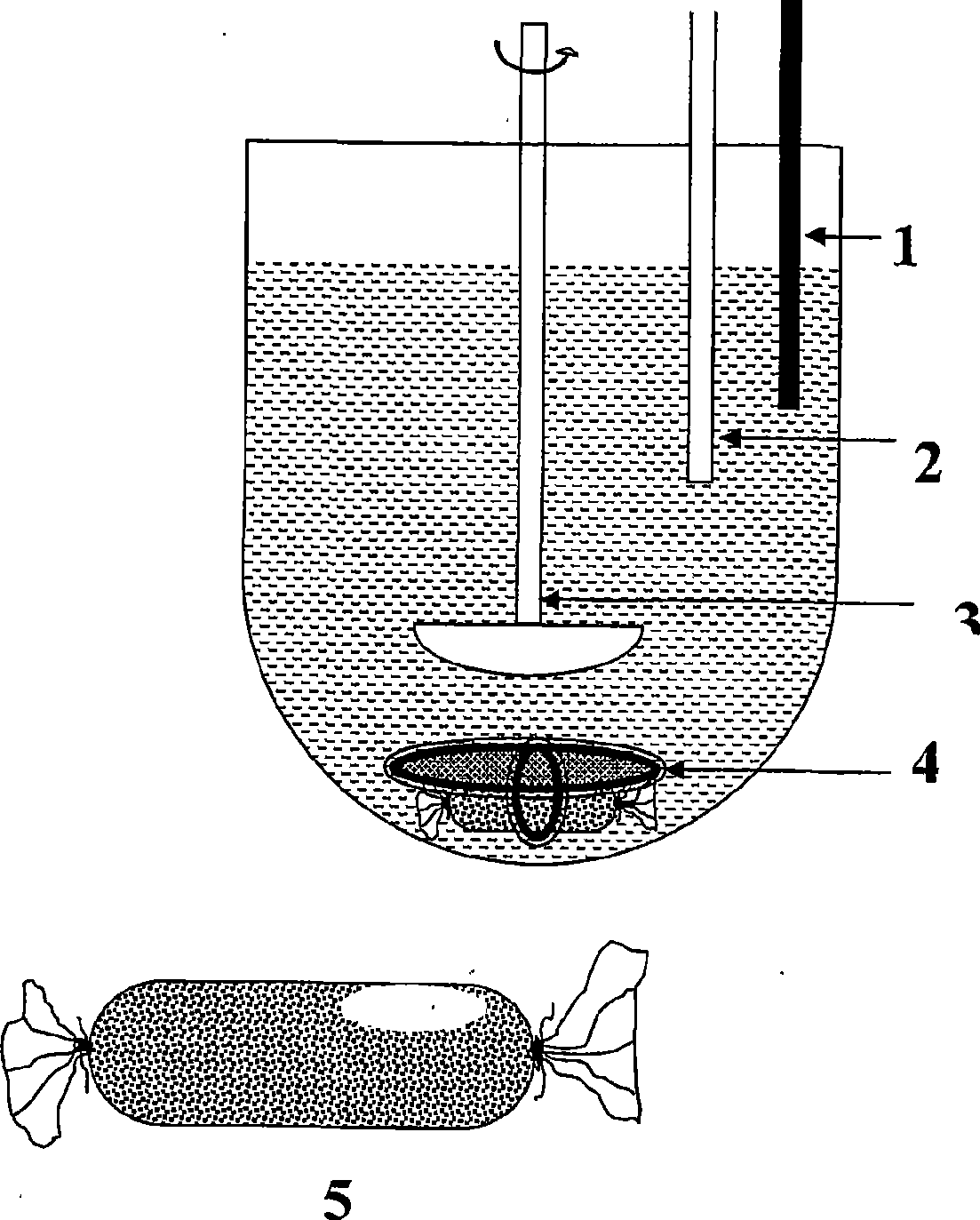
Image
Examples
Embodiment 1
[0119] Table 1
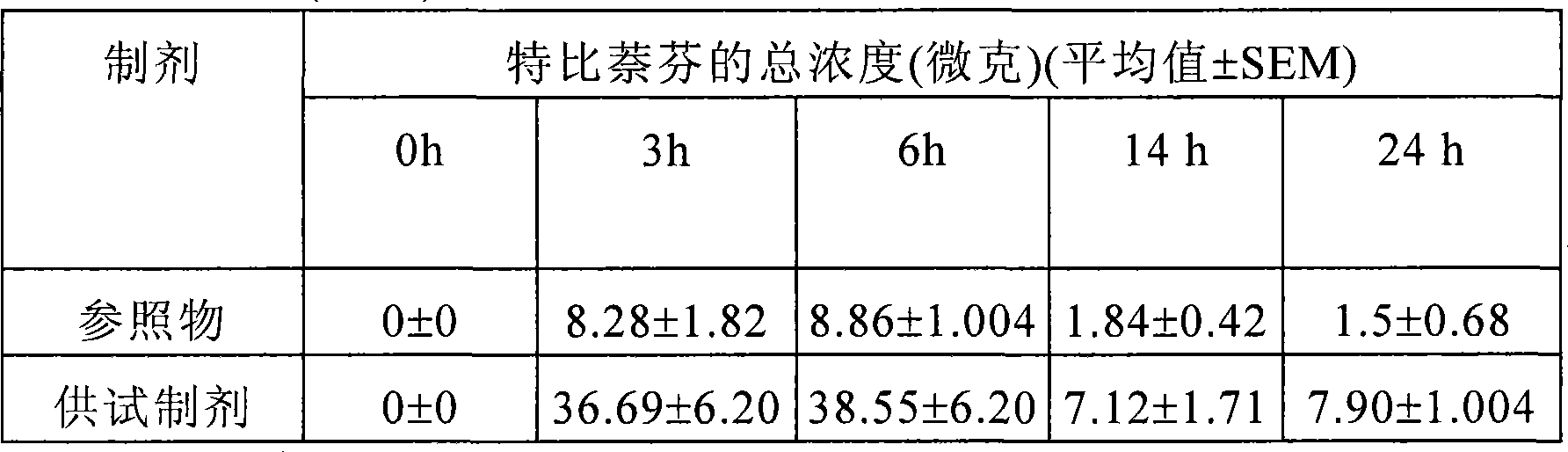
[0120] Element A B C D Terbinafine Hydrochloride 1.5g 1.5g 1.5g 1.5g Hydroxypropyl Cellulose
(HPC) 300mg 300mg 300.0mg -
Zinc nitrate hexahydrate 22.31g - 22.3g 22.3g Zinc acetate dihydrate - 25.5g - - Potassium hydroxide 8.4g 15.1g
(in 50g methanol) 8.4g 8.4g Methanol 75g 100g 60.0g 60.0g water 75g - 90.0g 90.0g
[0121] Method for 'A'
[0122] The metal oxide nanoparticles were obtained as follows: firstly zinc nitrate was dissolved in 50 g of water, and in another solution potassium hydroxide was dissolved in 25 g of water; after these two steps, terbinafine hydrochloride and hydroxyl Propylcellulose was dissolved in methanol to form solution 'A'. The previously prepared potassium hydroxide solution was added dropwise to solution 'A' under continuous stirring for about 20 minutes to form dispersion solution 'B'. The previously prepared zinc nitrate solution was a...
Embodiment 2
[0140] table 3
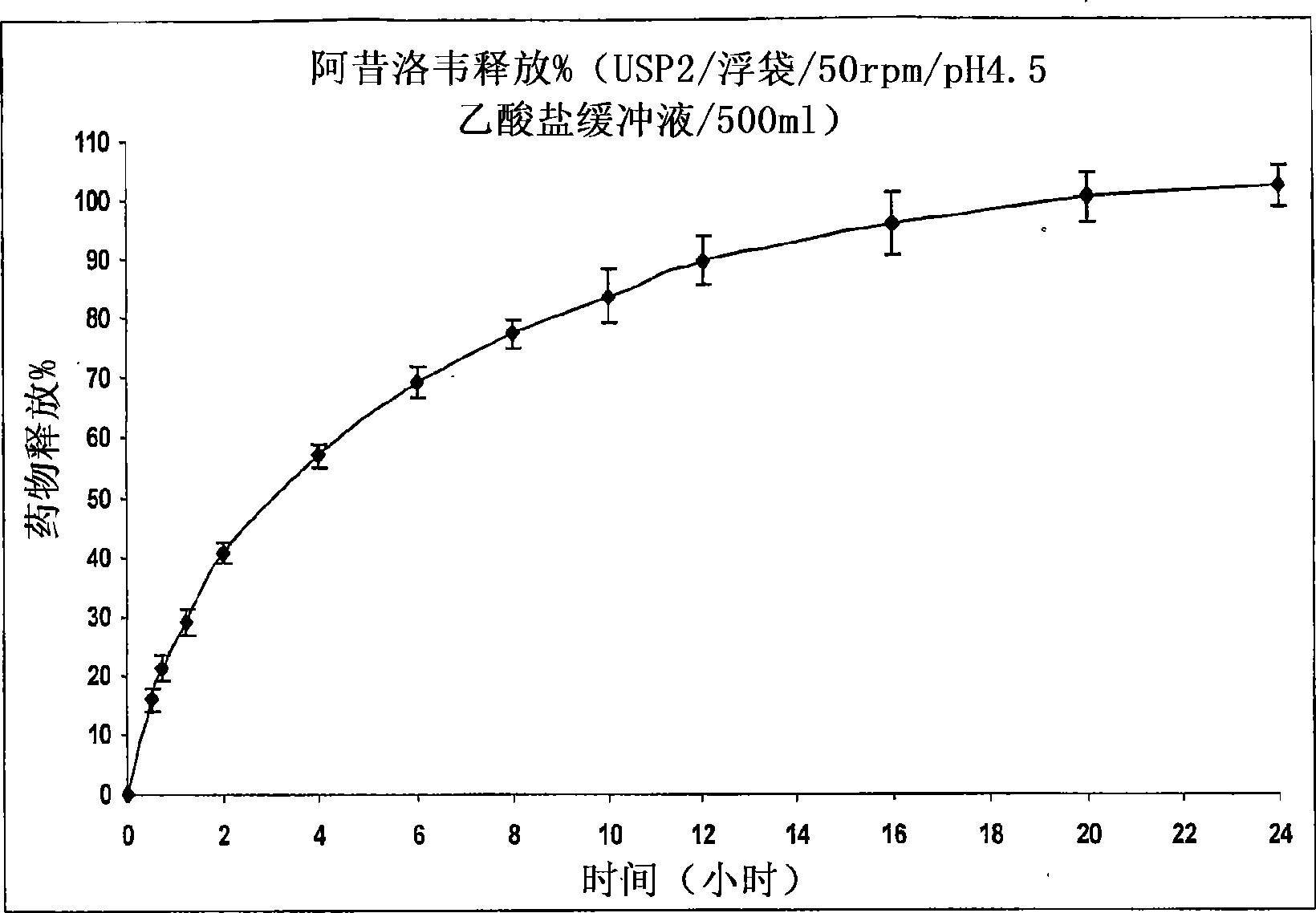
[0141] Element E F G H I Aciclovir 1.5g 1.5g 1.5g 1.5g 1.5g gum arabic - - 4.0g - - Mannitol - 44.0g - - - Potassium Hydroxide (KOH) 8.4g - - - - sodium hydroxide
(NaOH) _ 4.0g 4.0g 4.0g 4.0g Zinc nitrate hexahydrate 22.3g 14.87g 14.87g 14.87g 14.87g water 175g 200g 175g 150.0g 150.0g HPC-L 300mg 300.0mg 300.0mg 300.0mg -
[0142] method for E
[0143] Dissolve acyclovir together with potassium hydroxide in 50 g of water, then dissolve zinc nitrate in 50 g of water. Dissolve HPC independently in 75 grams of water. In addition, the three solutions were simultaneously mixed under stirring to obtain a white precipitate, which was lyophilized to obtain a white powder.
[0144] method for F
[0145] Mannitol was dissolved in 100 grams of water to form solution 'A'. Separately, acyclovir and NaOH were dissolved in 50 grams of water to form solution 'B'. In ...
Embodiment 33
[0167] table 5
[0168] Sample serial number Element J K L 1 gum arabic 4.0g _ - 2 pure water 75.0g - _ 3 Hydroxypropyl Cellulose 300.0mg 300.0mg _ 4 Zinc nitrate hexahydrate 14.87g 14.87g 14.87g 5 clindamycin phosphate 1.0g 1.0g 1.0g 6 pure water 50.0g 75.0g 75.0g 7 sodium hydroxide 4.0g 4.0g 4.0g 8 pure water 50.0g 75.0g 75.0g
[0169] Methods for J, K and L
[0170] Gum arabic was dissolved in 75.0 grams of purified water to form solution 'A', and hydroxypropyl cellulose and zinc nitrate were dissolved in purified water to form solution 'B'. In another step, the active molecule clindamycin was dissolved in an alkaline solution of sodium hydroxide to form solution C. Separately, solutions A, B, C (for method 'J') or solutions B, C (for method 'K') were mixed with continuous stirring at a controlled rate (0.2-0.5 ml / min) to form Dispersions. The dispersion was lyophilized to form a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com