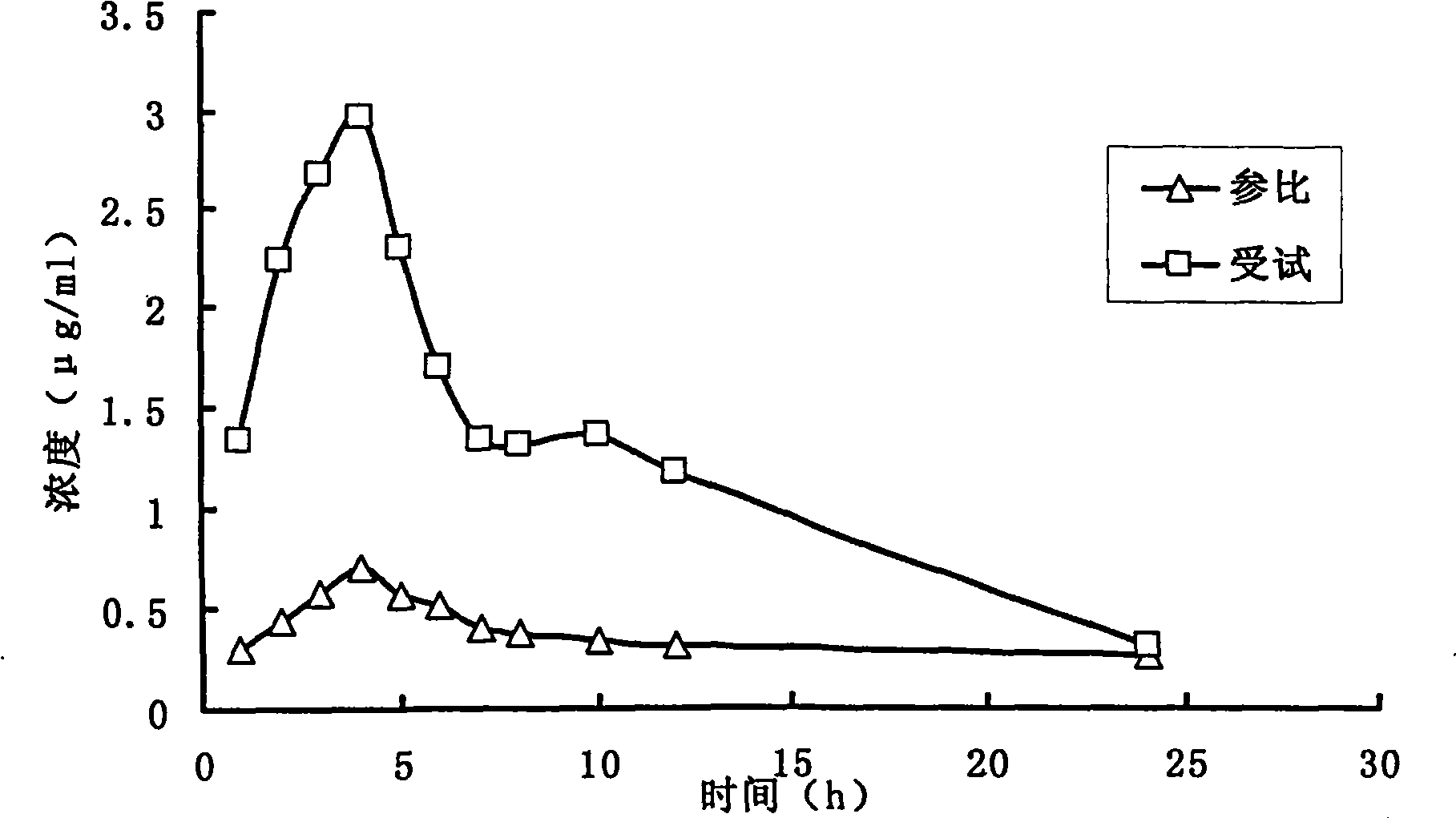
Fenofibrate medicament composition
A technology of fenofibrate and a composition, which is applied in the field of fibrate drugs, can solve the problems of low bioavailability of fibrate drugs, and achieve the effects of improving bioavailability, simple production process and reducing differences in bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Preparation of Fenofibrate Sustained Release Capsules
[0035] Take fenofibrate 2.4g, glyceryl behenate (compritol 888 ATO) 2.4g, polyethylene glycol 6000 (polyethylene glycol with a polymerization degree of 6000) 6g, polyethylene glycol laurate 0.6g, Polyoxyethylene hydrogenated castor oil (CremophorRH40) 0.4g.
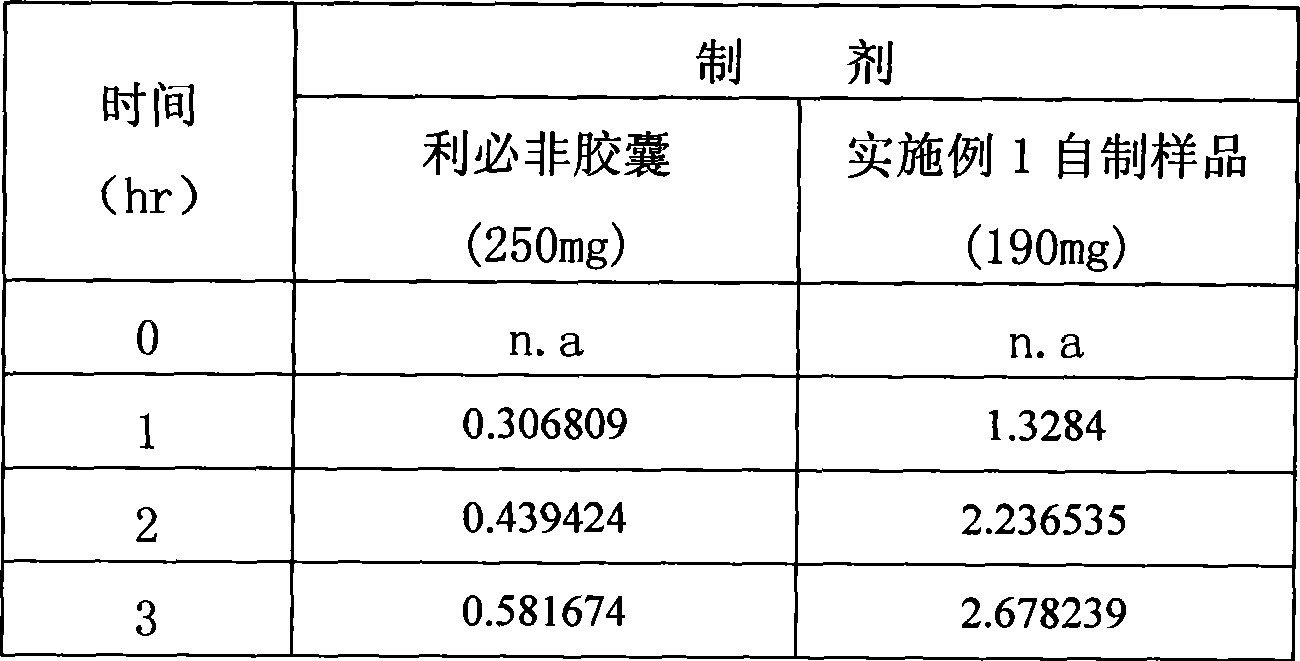
[0036] Add the above materials into a 50ml beaker, heat to about 90°C to melt, stir at low speed for about 10 minutes, and oscillate ultrasonically. Keep warm at 55°C and fill gelatin hard capsules or vegetable capsules. 190 mg per capsule (calculated as fenofibrate).
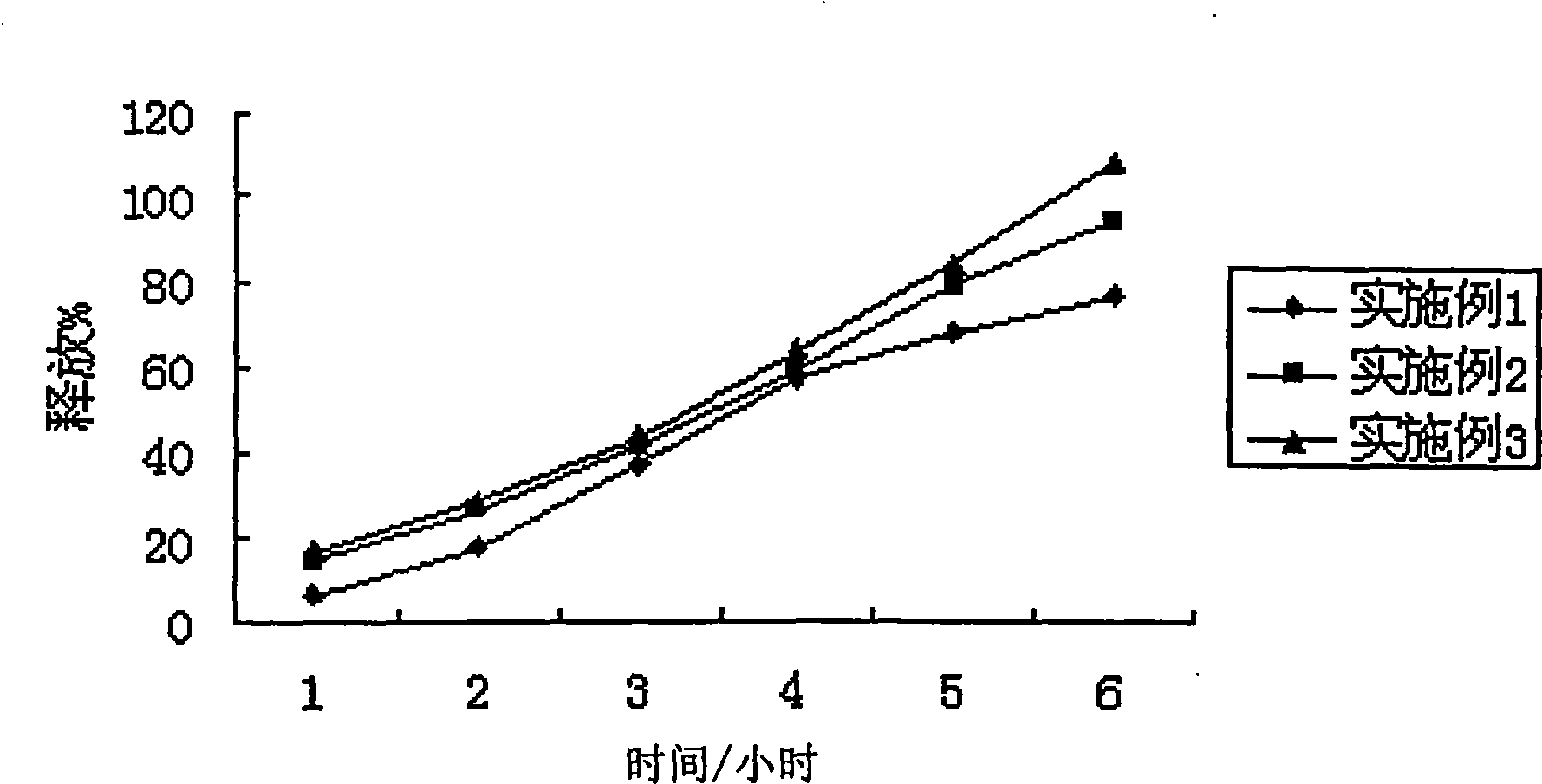
[0037] Get this product, according to the dissolution test method of European Pharmacopoeia, adopt the device of the second method of dissolution method, with 2% Tween 80 solution 1000ml as dissolution medium, rotating speed is 75 revolutions per minute, operate according to law. After 1, 4, and 6 hours, take 10ml of the solution and filter it, and add 10ml of the above-mentioned dissolution...
Embodiment 2
[0040] Preparation of sustained-release capsules
[0041] Take 2.4g of fenofibrate, 2.4g of glyceryl palmitostearate (Precirol ATO5), 4076g of poloxamer, 0.6g of polyethylene glycol laurate, and 0.4g of polyoxyethylene castor oil.
[0042] Add the above materials into a 50ml beaker, heat to about 90°C to melt, stir at low speed for about 10 minutes, and homogenize under high pressure. Keep warm at 55°C and fill gelatin hard capsules or vegetable capsules. 190 mg per capsule (calculated as fenofibrate).
[0043] Get this product, according to the dissolution test method of European Pharmacopoeia, adopt the device of the second method of dissolution method, with 2% Tween 80 solution 1000ml as dissolution medium, rotating speed is 75 revolutions per minute, operate according to law. After 1, 4, and 6 hours, take 10ml of the solution and filter it, and add 10ml of the above-mentioned dissolution medium in the operation container in time; accurately measure 2ml of the subsequent ...
Embodiment 3
[0046] Preparation of Fenofibrate Sustained Release Dropping Pills
[0047] Take fenofibrate 0.6g, glycerol palmitostearate 4.2g, polyethylene glycol 6000 (polyethylene glycol with a polymerization degree of 6000) 6g, ethylene glycol monoethyl ether 0.4g, polyoxyethylene castor Sesame oil 0.4g. 48 mg per capsule (calculated as fenofibrate).
[0048] Add the above materials into a 50ml beaker, heat to about 90°C to melt, and stir at a low speed for about 10 minutes. Insulate at 55°C, drop and cool to prepare sustained-release dropping pills.
[0049] Get this product, according to the dissolution test method of European Pharmacopoeia, adopt the device of the second method of dissolution method, take 2% Tween 80 solution 1000ml as the dissolution medium, and the rotating speed is 75 revolutions per minute, according to the law. After 1, 4, and 6 hours, take 10ml of the solution and filter it, and add 10ml of the above-mentioned dissolution medium in the operation container in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com