Ferro manganese composite oxides catalyst and preparation method and use thereof
A technology of composite oxides and catalysts, applied in metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, chemical instruments and methods, etc., can solve the problems of high cost, poor resistance to sulfur and water, etc. Achieve the effect of improving service life, low cost and strong resistance to sulfur poisoning
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
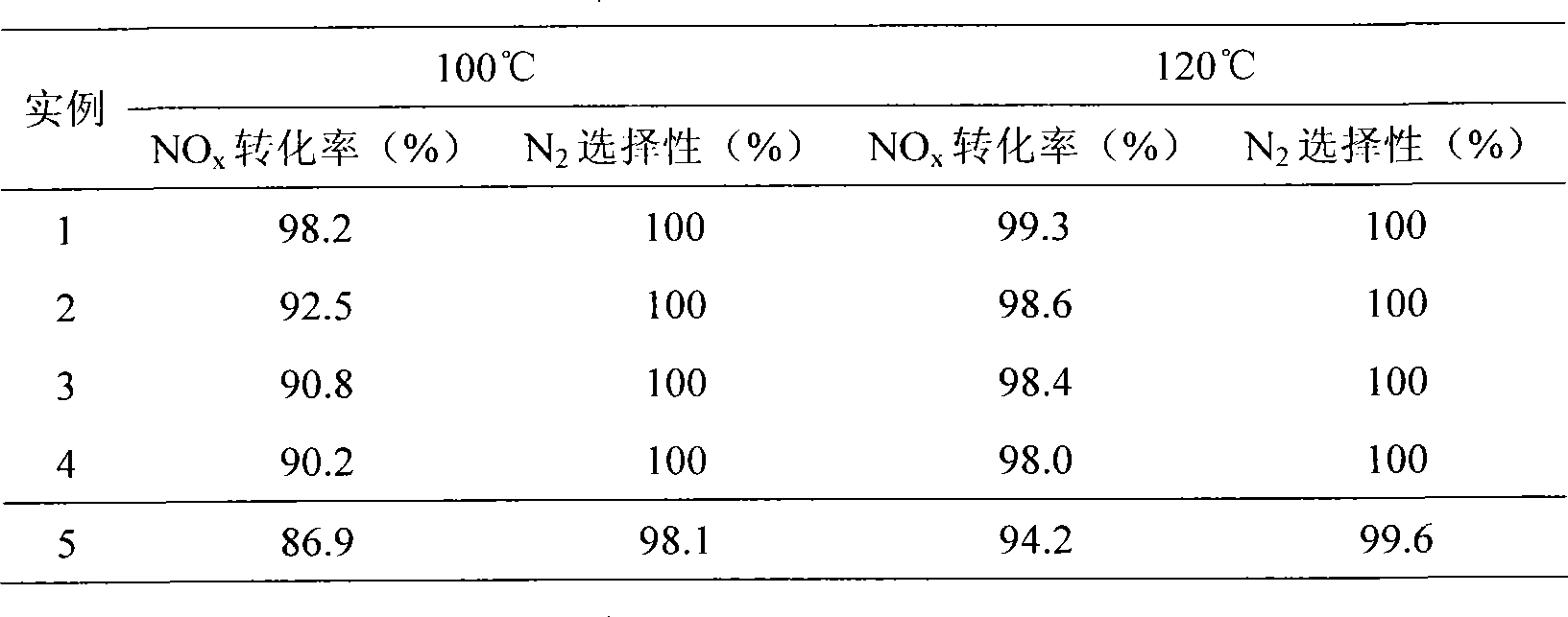
Embodiment 1
[0025] (1) Mix ferric nitrate and manganese nitrate with a molar ratio of metal elements of Fe:Mn=0.5:1, and add ferric nitrate and manganese nitrate with a molar ratio of 0.05:1 Nitrates or acetates of additive elements (a mixture of cobalt nitrate, zinc nitrate, cerium nitrate, vanadium acetate and copper acetate) to obtain mixed metal salts;
[0026] (2) adding a citric acid solution with a molar concentration of 0.2mol / L into the mixed metal salt obtained in step (1), the mol ratio of citric acid to the metal element in the mixed metal salt is 12:10, stirred for 6h, and mixed uniformly;
[0027] (3) drying at 100°C for 6 hours at a constant temperature to obtain a solid;
[0028] (4) calcining the solid obtained in step (3) in air for 8 hours at a calcination temperature of 400° C. to obtain a mixed oxide;
[0029] (5) Cool the calcined mixed oxide, press it into tablets, grind and sieve to obtain a catalyst product with a particle size of 60-100 mesh. This product inclu...
Embodiment 2
[0036] (1) Mix iron acetate and manganese acetate with a metal element molar ratio of Fe:Mn=6:1, and add metal elements (ie, iron element and manganese element) with a molar ratio of 0.01:1 to the mixture of iron acetate and manganese acetate. Nitrates or acetates of auxiliary elements (mixtures of cobalt acetate, zinc acetate, cerium nitrate, vanadium nitrate and copper nitrate) to obtain mixed metal salts;
[0037] (2) adding a citric acid solution with a molar concentration of 1mol / L into the mixed metal salt obtained in step (1), the molar ratio of the citric acid to the metal element in the mixed metal salt is 3: 1, stirred for 3h, and mixed uniformly;
[0038] (3) drying at 80°C for 24 hours at a constant temperature to obtain a solid;
[0039] (4) calcining the solid obtained in step (3) in air for 3 hours at a calcination temperature of 650° C. to obtain a mixed oxide;
[0040] (5) Cool the calcined mixed oxide, press it into tablets, grind and sieve to obtain a catal...
Embodiment 3
[0045] (1) Mix ferric nitrate and manganese acetate with a molar ratio of metal elements of Fe:Mn=3:1, and add metal elements (ie, iron and manganese elements) with a molar ratio of 0.08:1 to the mixture of ferric nitrate and manganese acetate. Nitrates or acetates of additive elements (a mixture of cobalt acetate, cerium nitrate, vanadium nitrate and copper nitrate) to obtain mixed metal salts;
[0046] (2) adding a citric acid solution with a molar concentration of 2mol / L in the mixed metal salt obtained in step (1), the mol ratio of the citric acid and the metal element in the mixed metal salt is 1: 1, stirred for 4h, and mixed uniformly;
[0047] (3) Drying at 130°C for 12 hours at a constant temperature to obtain a solid;
[0048] (4) calcining the solid obtained in step (3) in air for 4 hours at a calcination temperature of 350° C. to obtain a mixed oxide;
[0049] (5) Cool the calcined mixed oxide, press it into tablets, grind and sieve to obtain a catalyst product wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com