A pharmaceutical composition for oral comprising fenofibrate and preparation method thereof
A technology of fenofibrate and a composition is applied in the field of oral pharmaceutical compositions containing fenofibrate and the preparation thereof, and can solve the problems of reducing the yield of heat-labile drugs, thermal stability, adverse irritation of gastrointestinal mucosa, and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-5
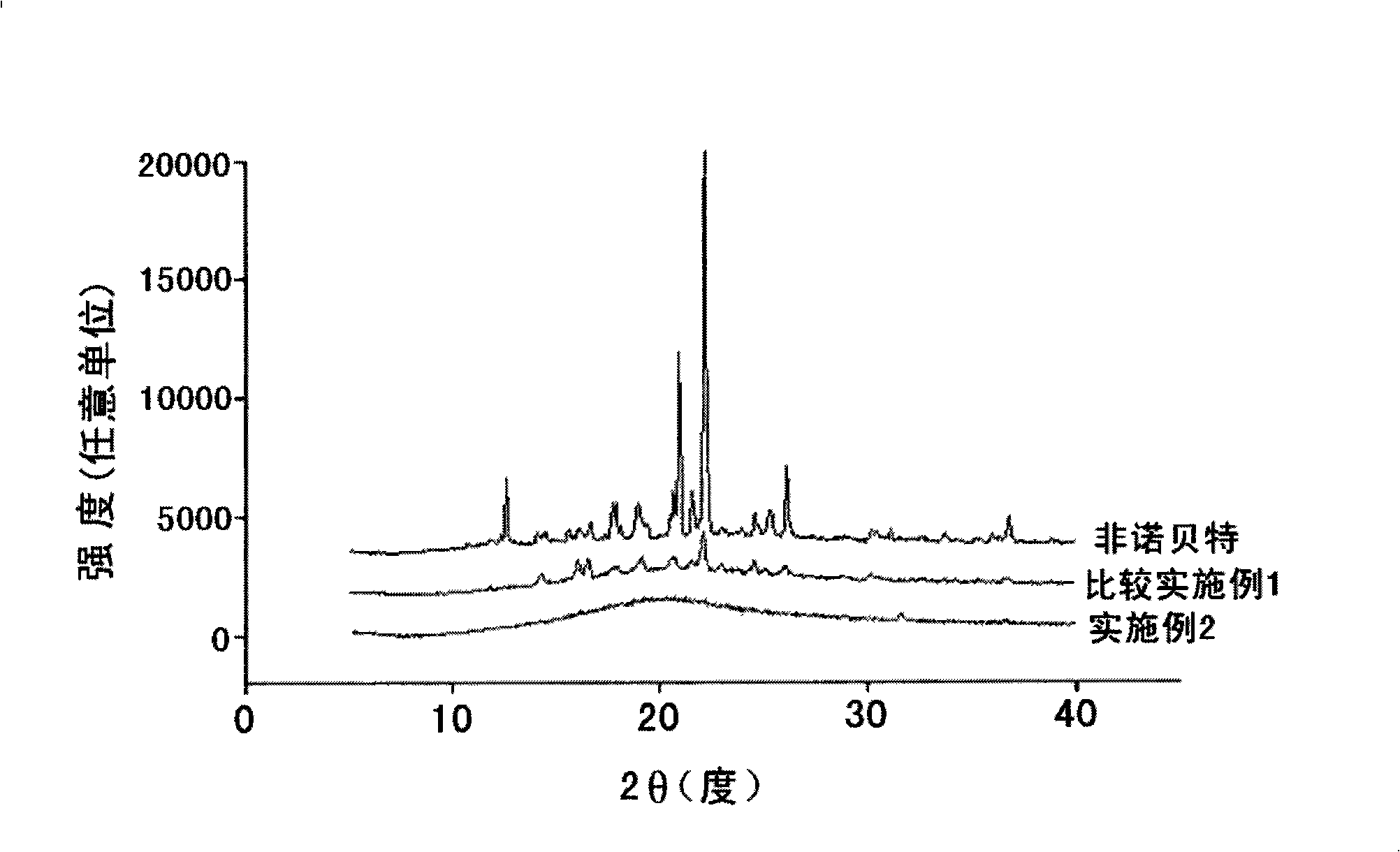
[0049] Preparation of solid dispersions according to the spray-drying method
[0050] The solid dispersion containing fenofibrate of the present invention was prepared according to the composition in Table 1.
[0051] Table 1
[0052]
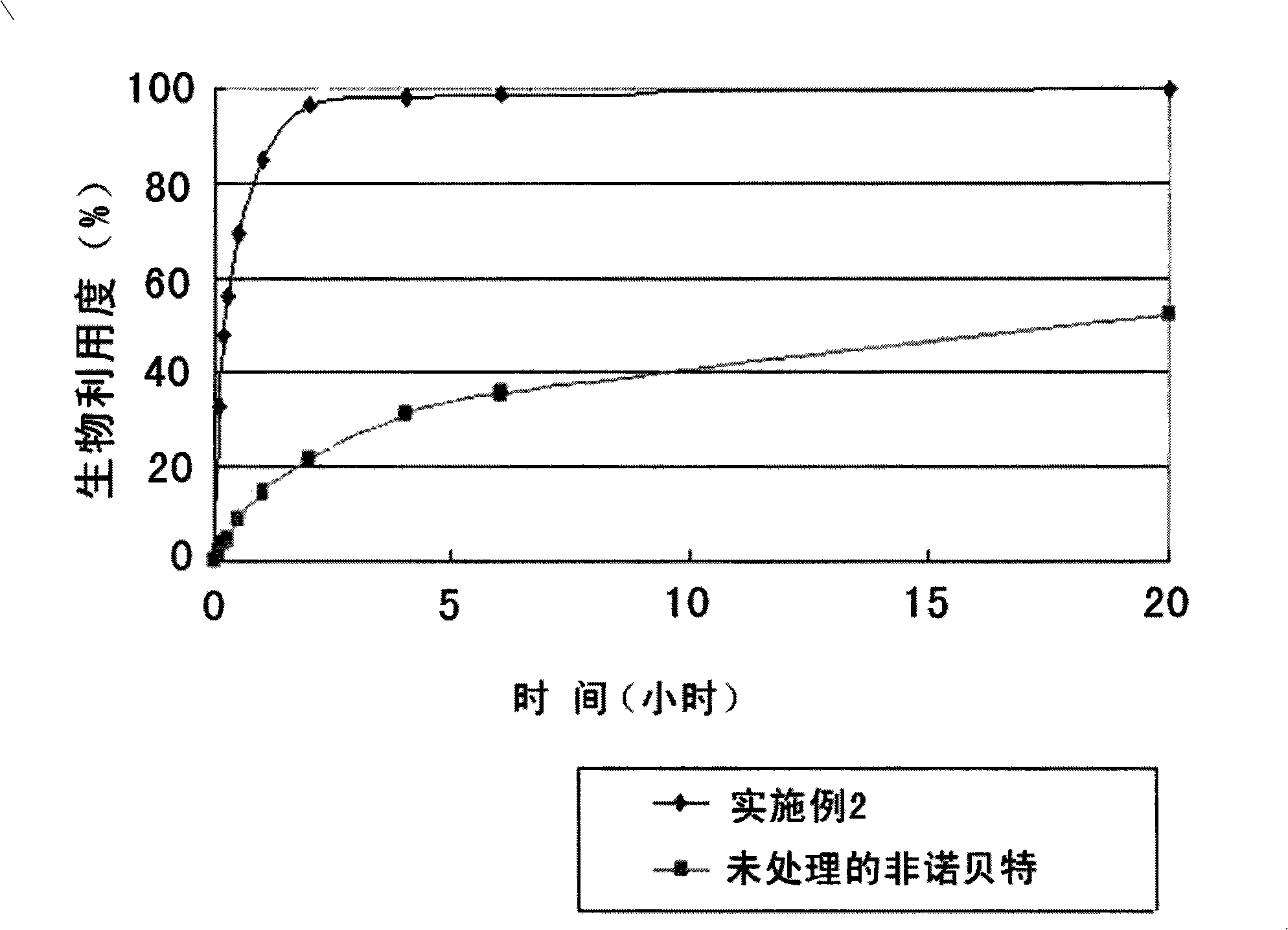
[0053] Fenofibrate was dissolved in a mixture of ethanol and dichloromethane at a volume ratio of 1:1, and hydroxypropylmethylcellulose was added as a water-soluble polymer to obtain a mixed solution of 8% (w / v). Poloxamer 188, Poloxamer 407, Cremophor RH-40, and soybean lecithin were added as surfactants to the mixed solution to prepare each solution. Each solution was spray-dried with a disc-type spray dryer to obtain a solid suspension in which fenofibrate was dispersed in hydroxypropylmethylcellulose. Spray drying is carried out under the following conditions: the feed temperature is 75-77°C, the combustion chamber temperature is 55-58°C, the rotary speed is 7,000-10,000rpm / min, and the flow rate is 16-20kg / h.
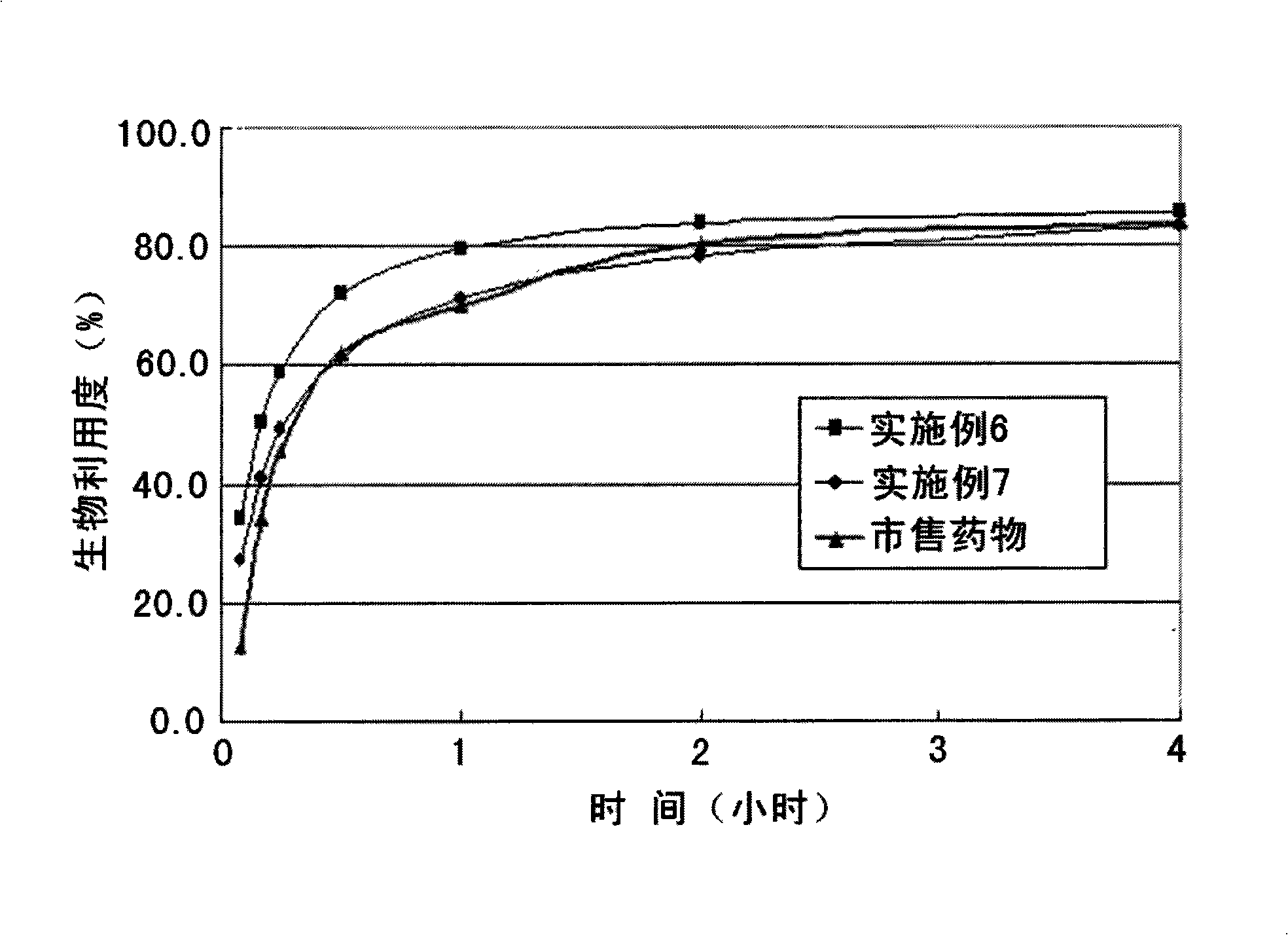
Embodiment 6-7
[0061] Preparation of Tablets Containing Fenofibrate Solid Dispersion
[0062] According to the dry granulation method, the solid dispersion of fenofibrate in Example 2 is made into granules, completely mixed with equal amounts of microcrystalline cellulose, lactose and cross-linked polyvinylpyrrolidone, and then mixed with colloidal anhydrous silicic acid as an enhancer. A plasticizer, magnesium stearate acts as a lubricant, and is then produced into tablets.
[0063] In Example 6, the fenofibrate solid dispersion was granulated with cross-linked polyvinylpyrrolidone as a disintegrant, while in Example 7, no cross-linked polyvinylpyrrolidone was added.
[0064] table 3
[0065]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Solubility | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com