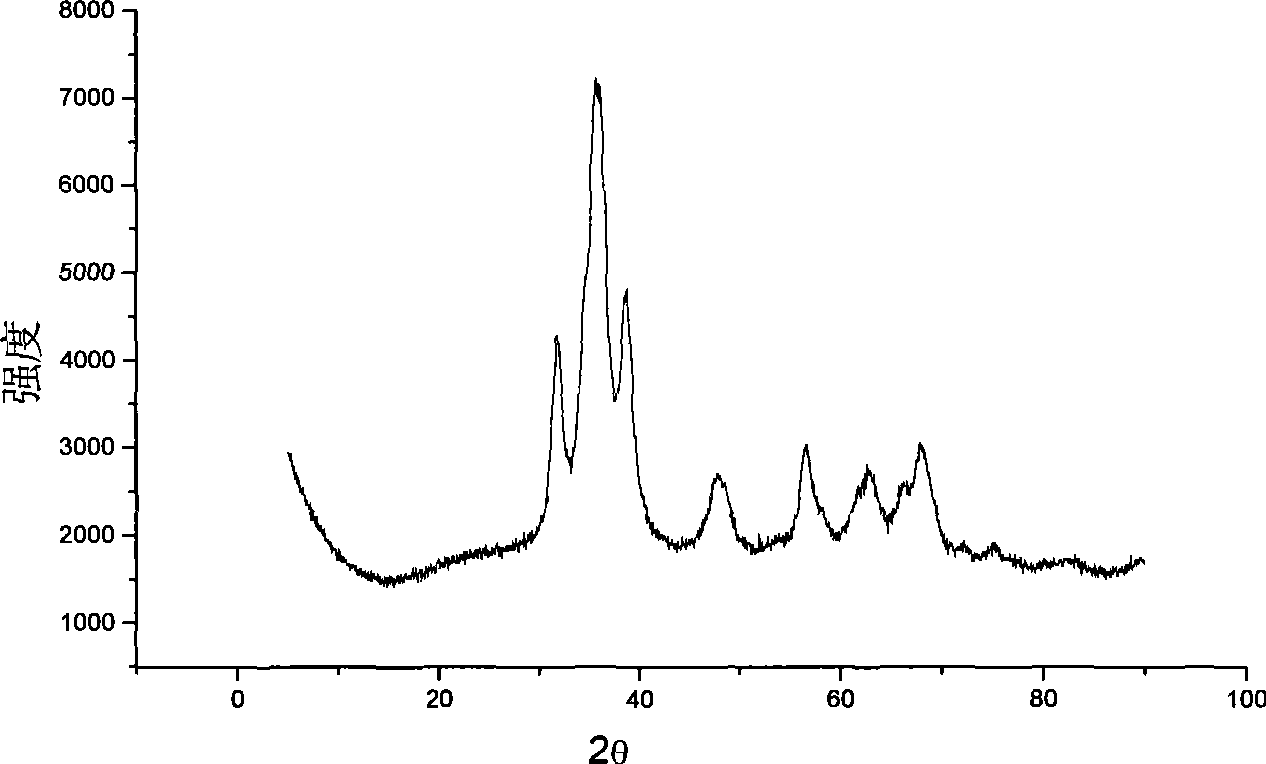
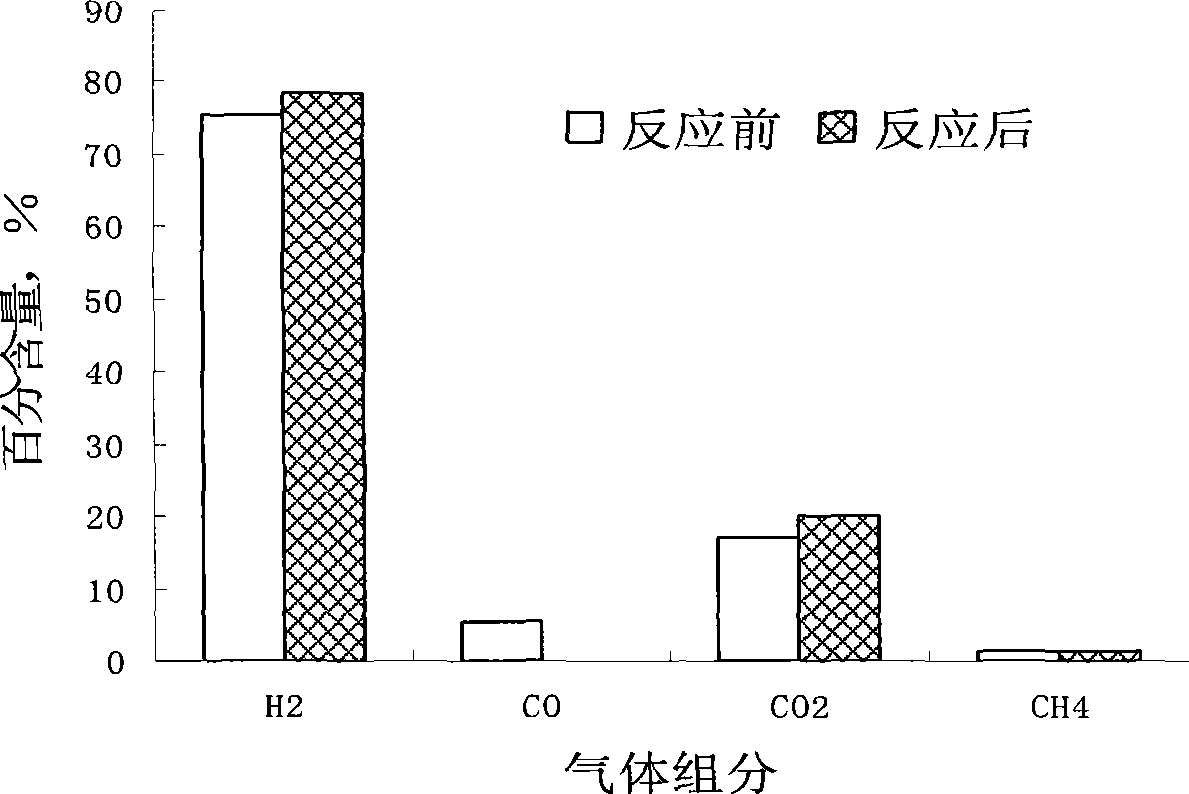
Catalyst for shifting carbon monoxide by water gas reaction in hydrogen-rich fuel gas and preparation method thereof
A technology of carbon monoxide and catalyst, which is applied in the field of water gas shift technology and catalyst, can solve the problems of difficult to meet the requirements of industrial production and high price, and achieve the effect of cheap raw materials, low manufacturing cost and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: CuO 40%, ZnO 40%, Al by mass percentage of oxide 2 o 3 The corresponding nitrate solution of 20% proportioning, take by weighing 46g Cu (NO 3 ) 2 ·3H 2 O, 56g Zn(NO 3 ) 2 ·6H 2 O and 45g Al(NO 3 ) 3 9H 2 O, dissolved in 500ml deionized water, stirred for 10 minutes, placed in an ultrasonic tank for ultrasonic treatment for 30 minutes, at the same time, weighed 59g Na 2 CO 3 Heat slowly in 500ml of deionized water until completely dissolved, co-precipitate the metal salt solution and alkali solution in the deionized bottom solution at a constant temperature of 60°C, adjust the pH value to 9 with 1M NaOH aqueous solution, stir and age for 1 hour, and dissolve the precipitate The solution was transferred to a constant temperature oil tank, and treated at a constant temperature of 80°C for 24 hours. During the precipitation process, the resulting precipitation was performed three times with deionized water and dried at 110°C for 10 hours. The mixture o...
Embodiment 2
[0025] Embodiment 2: CuO 35%, ZnO 25%, Al by oxide mass percentage 2 o 3 For the corresponding nitrate solution of 40% proportioning, weigh 39g Cu(NO 3 ) 2 ·3H 2 O, 33g Zn(NO 3 ) 2 ·6H 2 O and 86g Al(NO 3 ) 3 9H 2 O, dissolved in 500ml deionized water, stirred for 10 minutes, placed in an ultrasonic tank for ultrasonic treatment for 60 minutes, at the same time, weighed 65g Na 2 CO 3 Heat slowly in 500ml of deionized water until it is completely dissolved. Co-precipitate the metal salt solution and alkali solution in the deionized bottom solution at a constant temperature of 60°C. Adjust the pH value to 7 with 1M NaOH aqueous solution, stir and age for 3 hours, and dissolve the precipitated solution Transfer to a constant temperature oil tank, treat at a constant temperature of 60°C for 10 hours, use deionized water 5 times during the precipitation process, and dry at 80°C for 24 hours, roast the above-mentioned mixture at 500°C for 2 hours, and finally obtain a sol...
Embodiment 3
[0026] Embodiment 3: CuO 50%, ZnO 40%, Al by oxide mass percentage 2 o 3 The corresponding nitrate solution of 10% proportioning, weigh 63g Cu(NO 3 ) 2 ·3H 2 O, 59g Zn(NO 3 ) 2 ·6H 2 O and 18gAl(NO 3 ) 3 9H 2 O, dissolved in 500ml deionized water, stirred for 10 minutes, placed in an ultrasonic tank for ultrasonic treatment for 40 minutes, at the same time, weighed 55gNa2 CO 3 In 500ml of deionized water, heat slowly until completely dissolved, co-precipitate the metal salt solution and alkali solution in the constant temperature deionized bottom solution at 60°C, adjust the pH value to 9 with 1M NaOH aqueous solution, stir and age for 2 hours, and dissolve the precipitated solution Transfer to a constant temperature oil tank, treat at a constant temperature of 60°C for 24 hours, use deionized water for 4 times during the precipitation process, and dry at 100°C for 16 hours, and roast the above-mentioned mixture at 400°C for 4 hours, and finally obtain a solid Crush...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com