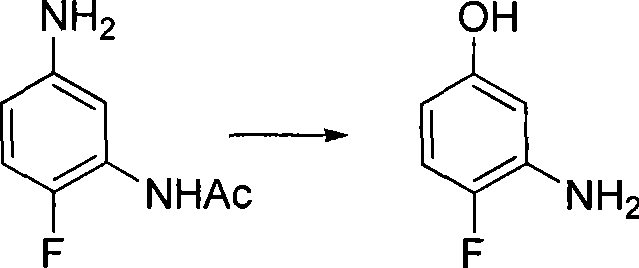
Method for preparing 3-amino-4-fluorophenol
A technology of fluorophenol and fluorophenol ester, which is applied in the field of preparation of 3-amino-4-fluorophenol, can solve the problems of difficult preparation of raw materials, difficult reaction control, and low selectivity, and achieves less side reactions, low cost, The effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The preparation of embodiment 1 3-amino-4-fluorophenol
[0042] (1) Preparation of 2-bromo-4-fluorophenol:
[0043] Add 200g (1.785mol) of p-fluorophenol and 300ml of dichloroethane into a 2L reaction bottle, and after mixing, add dropwise a mixture of 300g (1.875mol) of bromine and 150ml of dichloroethane at 5°C to 10°C. Afterwards, keep warm for half an hour. Then add 33g (0.26mol) sodium sulfite and 200ml water mixed solution, stir for 30min, let stand to separate layers, take the organic layer, wash with mixed lye (10%NaOH / 20%NaHCO 3) to neutral, dried over anhydrous magnesium sulfate, and the solvent was evaporated by a rotary evaporator to obtain 343 g (1.688 mol) of yellow liquid 2-bromo-4-fluorophenol. Molar yield: 95%, the purity determined by gas chromatography is 94%. The boiling point is 145°C / 20mmHg.
[0044] (2) Preparation of ethyl 2-bromo-4-fluorophenoxyformate
[0045] Add 74.3g of sodium hydroxide (1.857mol) and 675ml of water into a 2L reaction f...
Embodiment 2
[0052] Example 2 Preparation of 3-amino-4-fluorophenol
[0053] (1) Preparation of 2-bromo-4-fluorophenol
[0054] Add 200g (1.785mol) of p-fluorophenol and 361g (2.142mol) of 48% hydrobromic acid into a 2L reaction bottle, mix and add 269.5g (2.142mol) of 27% H 2 o 2 After dripping, heat preservation reaction for half an hour, then add 45g (0.357mol) sodium sulfite, stir for 30min, let stand for layering, take the organic layer and use mixed lye (10%NaOH / 20%NaHCO 3 ) to neutral, and dried over anhydrous magnesium sulfate to obtain a yellow liquid. The boiling point is 145°C / 20mmHg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com