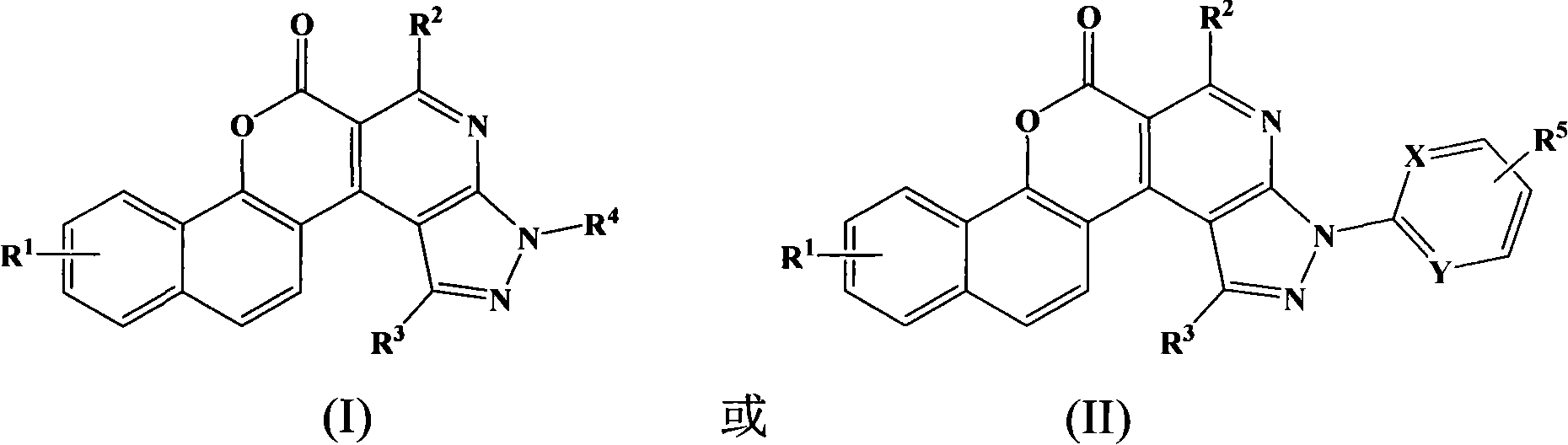
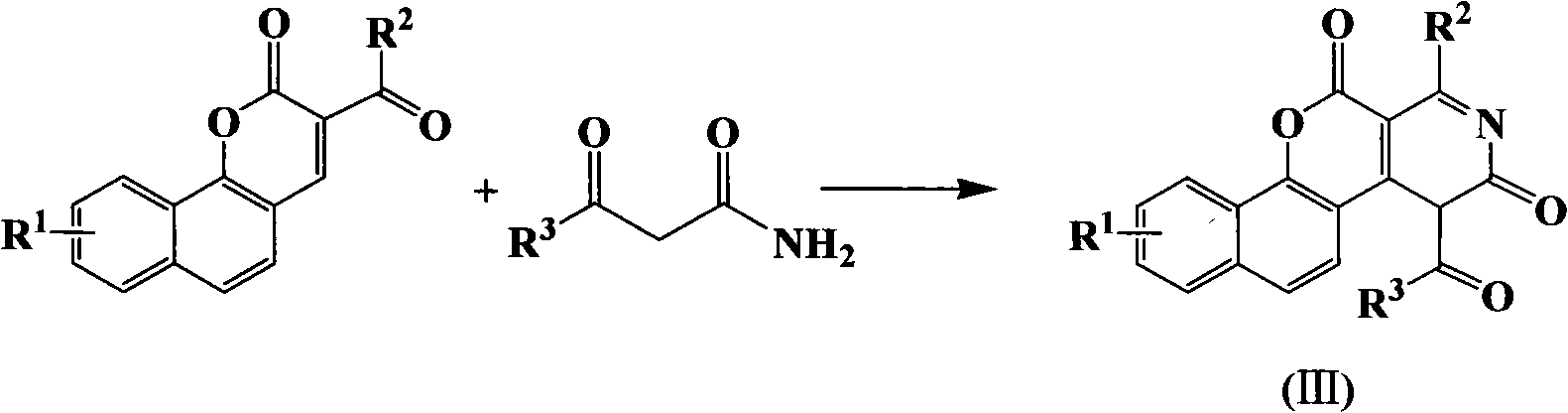
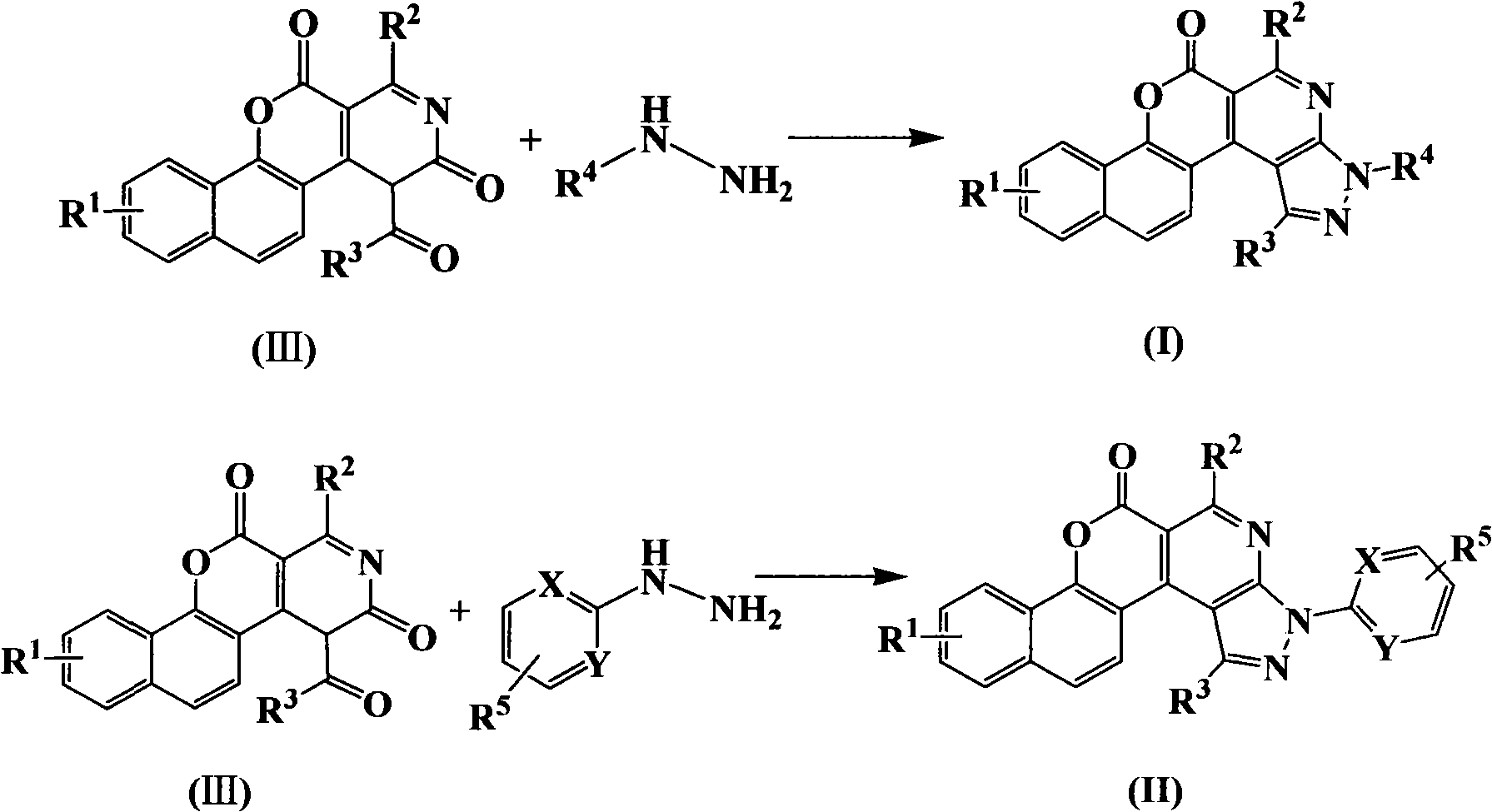
Pyrazoline naphthyridine benzocoumarin fluorescent dye derivatives, synthesis method of same, and application of same
A technology of benzocoumarin and pyrazoline pyridine, applied in the field of laser dyes and fluorescent dyes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Synthesis of Benzocoumarin Derivative (I)-1
[0052]
[0053] The synthesis of 9-N, N-diethylaminobenzocoumarin aldehyde is referred to in the literature (J.Org.Chem.2007, 72, 2088-2096; J.Med.Chem.2004, 47, 6349).
[0054] Dissolve 2.95g (0.01mol) of 9-N,N-diethylaminobenzocoumarin aldehyde and 1.01g (0.01mol) of acetoacetamide in 30mL of ethanol, add 0.5mL of triethylamine, 90°C Heated to reflux for 8 hours, solid precipitated after cooling, and filtered to obtain 1.94 g of (III)-1 intermediate, with a yield of 51.6%. Compound (III)-1 can be directly used in the next reaction without purification.
[0055] Dissolve 1.88g (0.005mol) of compound (III)-1 and 0.23g (0.005mol) of methylhydrazine in 30mL of ethanol, heat and reflux at 90°C for 6 hours, and precipitate a solid after cooling, recrystallize after filtration to obtain 1.05g of benzo Coumarin derivative (I)-1, yield 54.4%.
[0056] EI-MS, m / e, 387.2[M+1] + .λ ab. max / nm(CH 2 Cl 2 ) = 470nm, λ em ma...
Embodiment 2
[0058] Synthesis of Benzocoumarin Derivative (I)-2
[0059]
[0060] The synthesis of 9-N, N-diethylaminobenzocoumarin aldehyde and intermediate (III)-1 is the same as in Example 1.
[0061] Dissolve 1.88g (0.005mol) of compound (III)-1 and 0.74g (0.005mol) of phenylhydrazine in 30mL of ethanol, heat to reflux at 90°C for 6 hours, and precipitate a solid after cooling, recrystallize after filtration to obtain 1.15g of benzoin Soybean derivative (I)-2, the yield is 55.6%.
[0062] EI-MS, m / e, 415.2[M+1] + .λ ab. max / nm(CH 2 Cl 2 )=465nm,λ em max / nm(CH 2 Cl 2 ) = 580nm, Φ f e = 0.93.
Embodiment 3
[0064] Synthesis of Benzocoumarin Derivative (II)-1
[0065]
[0066] The synthesis of 9-N, N-diethylaminobenzocoumarin aldehyde and intermediate (III)-1 is the same as in Example 1.
[0067] Dissolve 1.88g (0.005mol) of compound (III)-1 and 0.54g (0.005mol) of phenylhydrazine in 30mL of ethanol, heat to reflux at 90°C for 6 hours, and precipitate a solid after cooling, recrystallize after filtration to obtain 1.26g of benzoin Soybein derivative (II)-1, the yield is 56.3%.
[0068] EI-MS, m / e, 449.2[M+1]+ .λ ab. max / nm(CH 2 Cl 2 ) = 475nm, λ em max / nm(CH 2 Cl 2 ) = 588nm, Φ f e = 0.95.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com