Method for separating and extracting isostearic acid from monomer acid
A technology of isostearic acid and isostearic acid ester, which is applied in the field of separation and extraction of isostearic acid, can solve the problems of low antioxidant performance, high iodine value, and easy to darken product color, so as to avoid corrosion and improve the Efficiency and service life effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
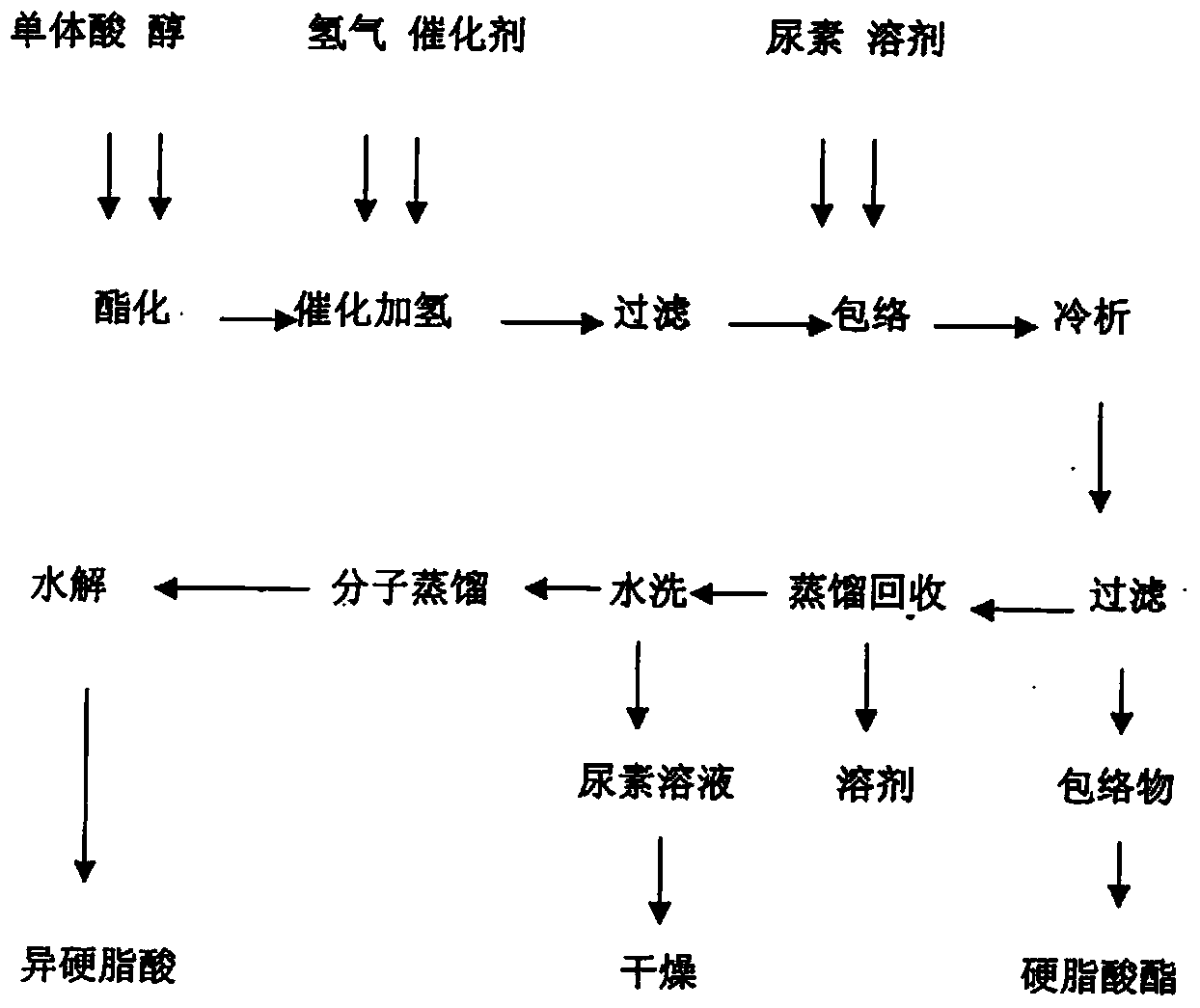
[0022] A method for separating and extracting isostearic acid from monomeric acid includes the following steps:
[0023] (1) Monomer esterification
[0024] The monomer acid and anhydrous methanol are pumped into the pipeline reactor with a pressure pump at a volume ratio of 1:1, and the feed of monomer acid and methanol is maintained at a pressure of 2.0 MPa and a reaction temperature of 160-240°C. The speed is 50L / h, and the reaction is 19 hours. When the acid value of the reactant drops below 2mgKOH / g, the methyl esterification reaction is completed, vacuum is applied, and water and methanol are distilled off at 0.07MPa and temperature 35-65°C to obtain Monomer acid methyl ester. The methanol can be reused after dehydration.
[0025] (2) Catalytic hydrogenation of monomeric acid esters
[0026] Add the monomeric acid methyl ester obtained in (1) into the hydrogenation reaction kettle, put in the Raney nickel catalyst with 0.3% of the monomer acid weight, seal the reaction kettle,...
Embodiment 2
[0034] A method for separating and extracting isostearic acid from monomeric acid includes the following steps:
[0035] (1) Monomer esterification
[0036] The monomer acid and anhydrous ethanol are pumped into the pipeline reactor with a pressure pump at a volume ratio of 1:1.1, and the monomer acid and ethanol are maintained at a pressure of 2.5 MPa and a reaction temperature of 170-250°C. The speed is 45L / h, and the reaction is 20 hours. When the acid value of the reactant drops below 2mgKOH / g, the esterification reaction is completed, vacuum is applied, and the ethanol is distilled off at 0.06MPa and temperature 50-70℃ to obtain monomer acid Ethyl ester. The recovered ethanol can be reused after dehydration.
[0037] (2) Catalytic hydrogenation of monomeric acid ethyl ester
[0038] Add the product obtained in (1) into the hydrogenation reactor, put in the Raney nickel catalyst of 0.3% by weight of monomer acid, seal the reactor, and evacuate. When the pressure in the reactor r...
Embodiment 3
[0046] A method for separating and extracting isostearic acid from monomeric acid includes the following steps:
[0047] (1) Monomer esterification
[0048] The monomer acid and anhydrous isopropanol are pumped into the pipeline reactor with a pressure pump at a volume ratio of 1:1.1, and the monomer acid and isopropanol are maintained at a pressure of 3.0MPa and a reaction temperature of 160-240℃. The feed rate of the alcohol is 40L / h, and the reaction is 21 hours. When the acid value of the reactant drops below 2mgKOH / g, the esterification reaction is completed, vacuum is applied, and isopropyl is distilled off at 0.07MPa and temperature 50-70°C. Alcohol to obtain monomeric acid isopropyl ester.
[0049] (2) Catalytic hydrogenation of monomeric acid esters
[0050] Put the isopropyl monomer acid obtained in (1) into the hydrogenation reactor, put in the Raney nickel catalyst of 0.3% by weight of isopropyl monomer acid, seal the reactor and evacuate. When the pressure in the kettle ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com