Method for preparing barium-cobalt-iron-niobium composite oxide with a low-temperature self-propagating combustion method
A self-propagating combustion and composite oxide technology, which is used in the preparation of ceramic oxygen-permeable membrane materials and functional materials, can solve the problems of small interval between temperature and material melting temperature, long time, and high energy consumption, which is conducive to sintering, The effect of reducing synthesis temperature and saving energy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
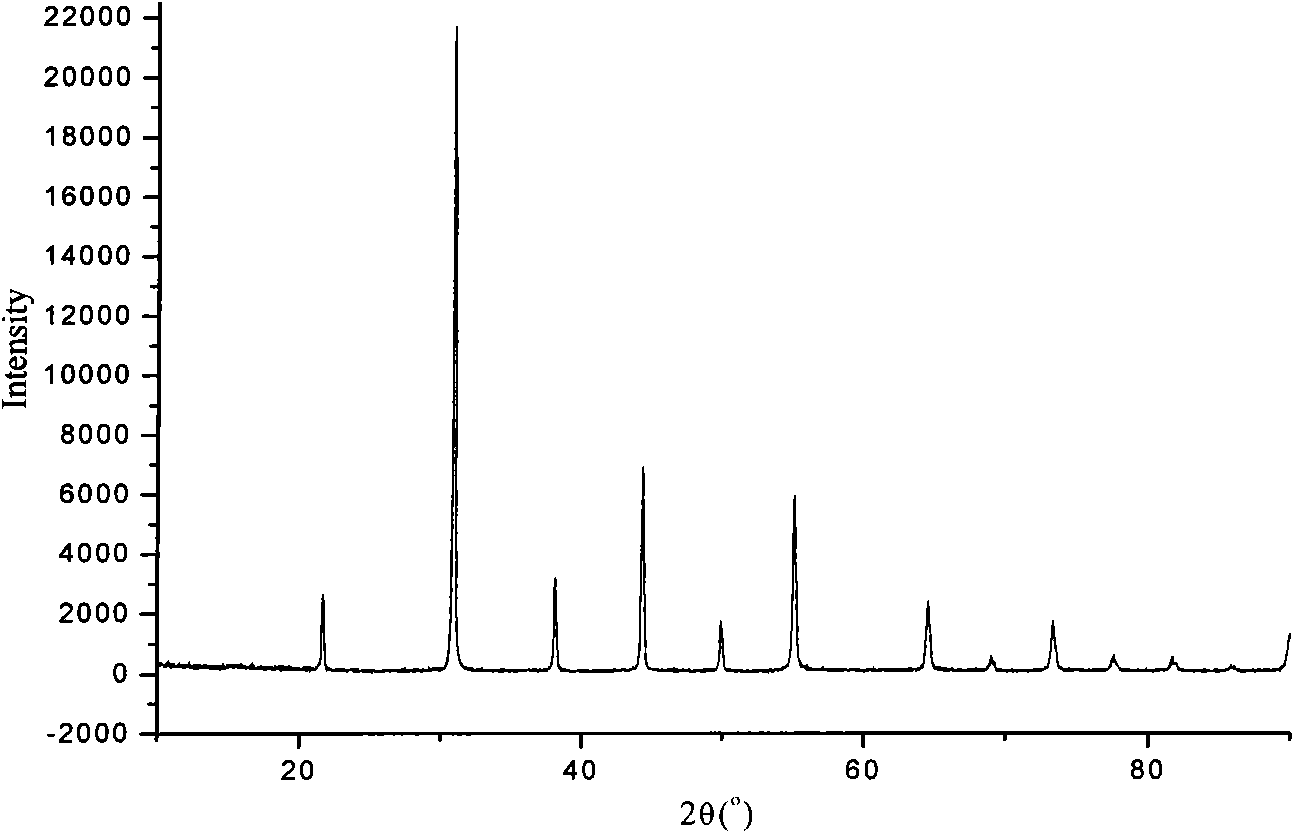
Image
Examples
Embodiment 1
[0029] 1) Mole ratio Ba(NO 3 ) 2 : Co(NO 3 ) 2 ·6H 2 O:Fe(NO 3 ) 3 9H2 The ratio of O=1:0.7:0.2, weigh Ba(NO 3 ) 2 13.067 g, Co(NO 3 ) 2 ·6H 2 O 10.186 g, Fe(NO 3 ) 3 9H 2 O 4.04 grams, dissolved in 300ml deionized water;
[0030] 2) take by weighing citric acid (C 6 h 8 o 7 ·H 2 O) 21.014 grams were mixed with the solution obtained in the first step, and stirred for 10 minutes under heating in a water bath at 80° C. to obtain a dark red clear solution;
[0031] 3) Press Ba 1.0 co 0.7 Fe 0.2 Nb 0.1 o 3-δ The stoichiometric ratio weighs Nb 2 o 5 0.665 g, add excess HF acid and heat it in a water bath at 80°C to promote its complete reaction. After cooling with ice water, add ammonia water dropwise to the obtained clear liquid, and adjust the pH value to 9. When the precipitation is complete, the precipitate is suction filtered and washed After several times, the filter cake was transferred to a beaker and added with 0.01mol citric acid (C 6 h 8 o 7 ·...
Embodiment 2
[0035] 1) synchronously with 1) of embodiment 1;
[0036] 2) take by weighing citric acid (C 6 h 8 o 7 ·H 2 O) 12.608 g was mixed with the solution obtained in the first step, and stirred for 5 minutes under heating in a water bath at 80° C. to obtain a red clear solution;
[0037] 3) 3) in step with embodiment 1;
[0038] 4) Synchronize with 4) of embodiment 1;
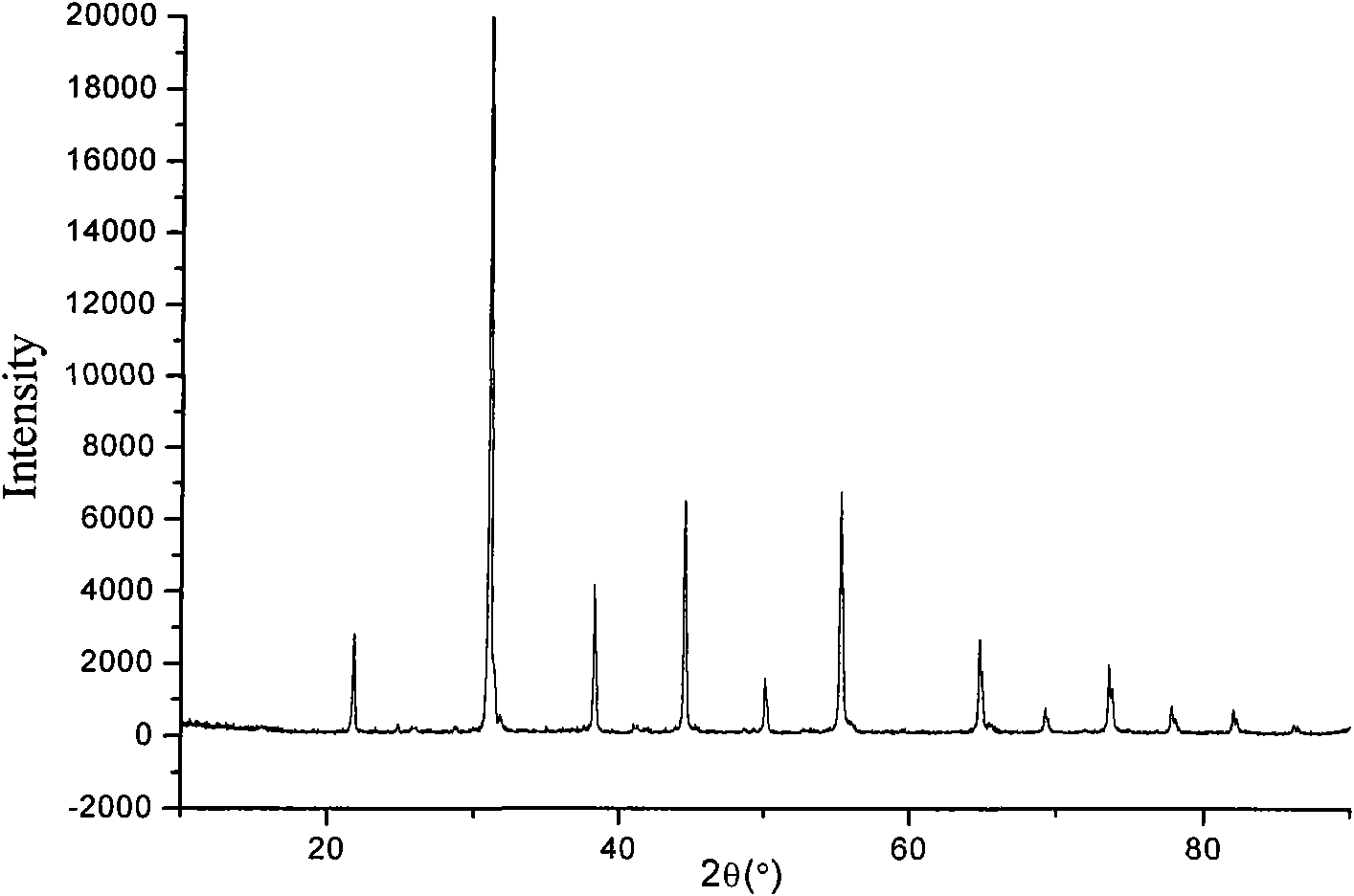
[0039] 5) step 5) of embodiment 1, the XRD collection of illustrative plates of synthetic product sees figure 2 , no impurity phase was seen.
Embodiment 3
[0041] 3) 1) in step with embodiment 1;
[0042] 4) take by weighing citric acid (C 6 h 8 o 7 ·H 2 O) 42.028 g was mixed with the solution obtained in the first step, and stirred for 5 minutes under heating in a water bath at 80° C. to obtain a red clear solution;
[0043] 3) 3) in step with embodiment 1;
[0044] 4) Synchronize with 4) of embodiment 1;
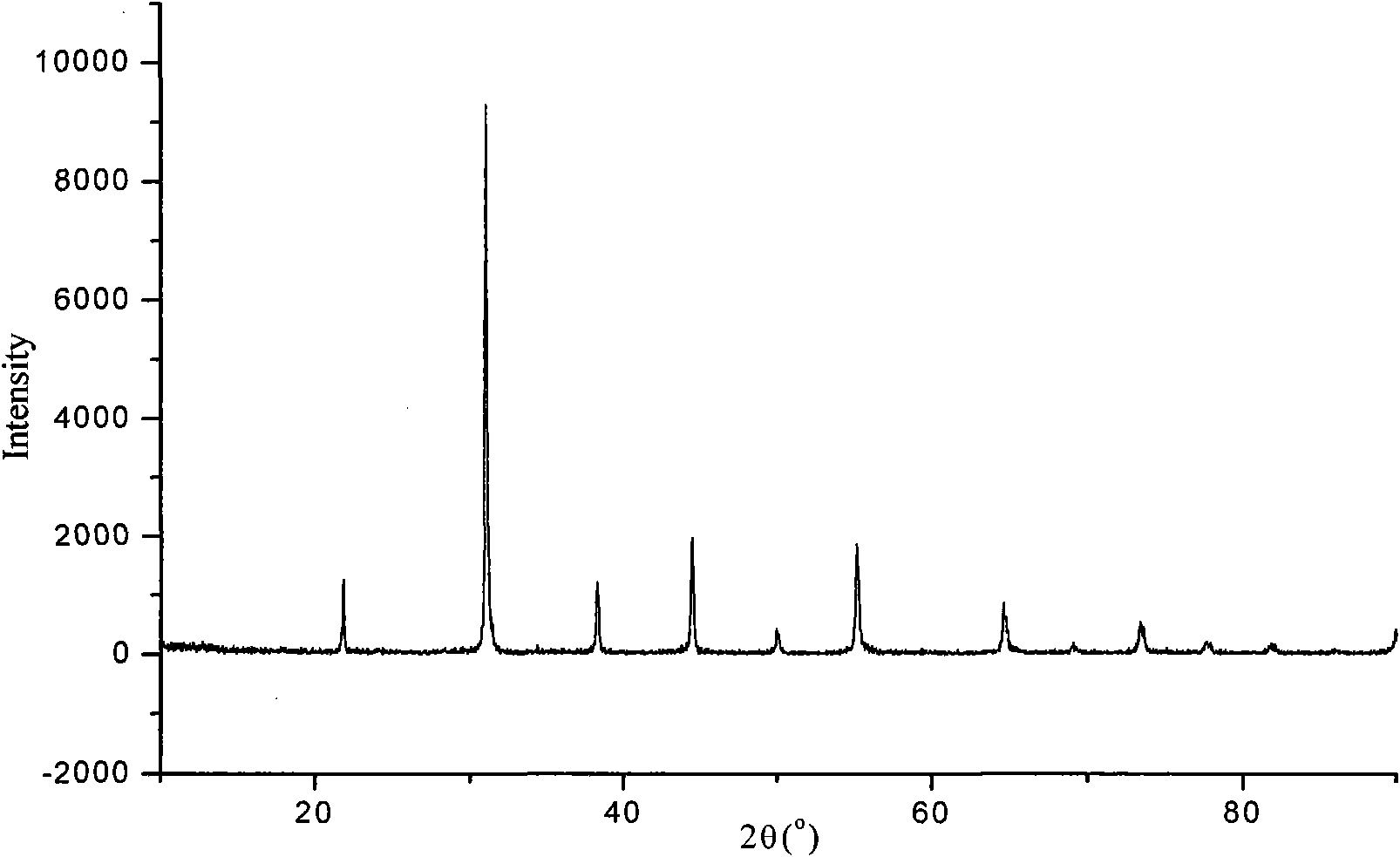
[0045] 5) step 5) of embodiment 1, the XRD collection of illustrative plates of synthetic product sees image 3 , no impurity phase was seen.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com