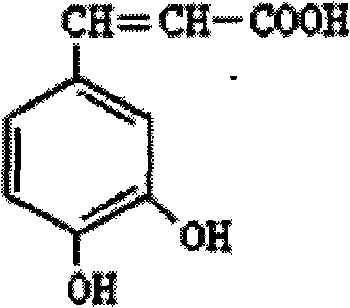
Caffeic acid composition and preparation method thereof
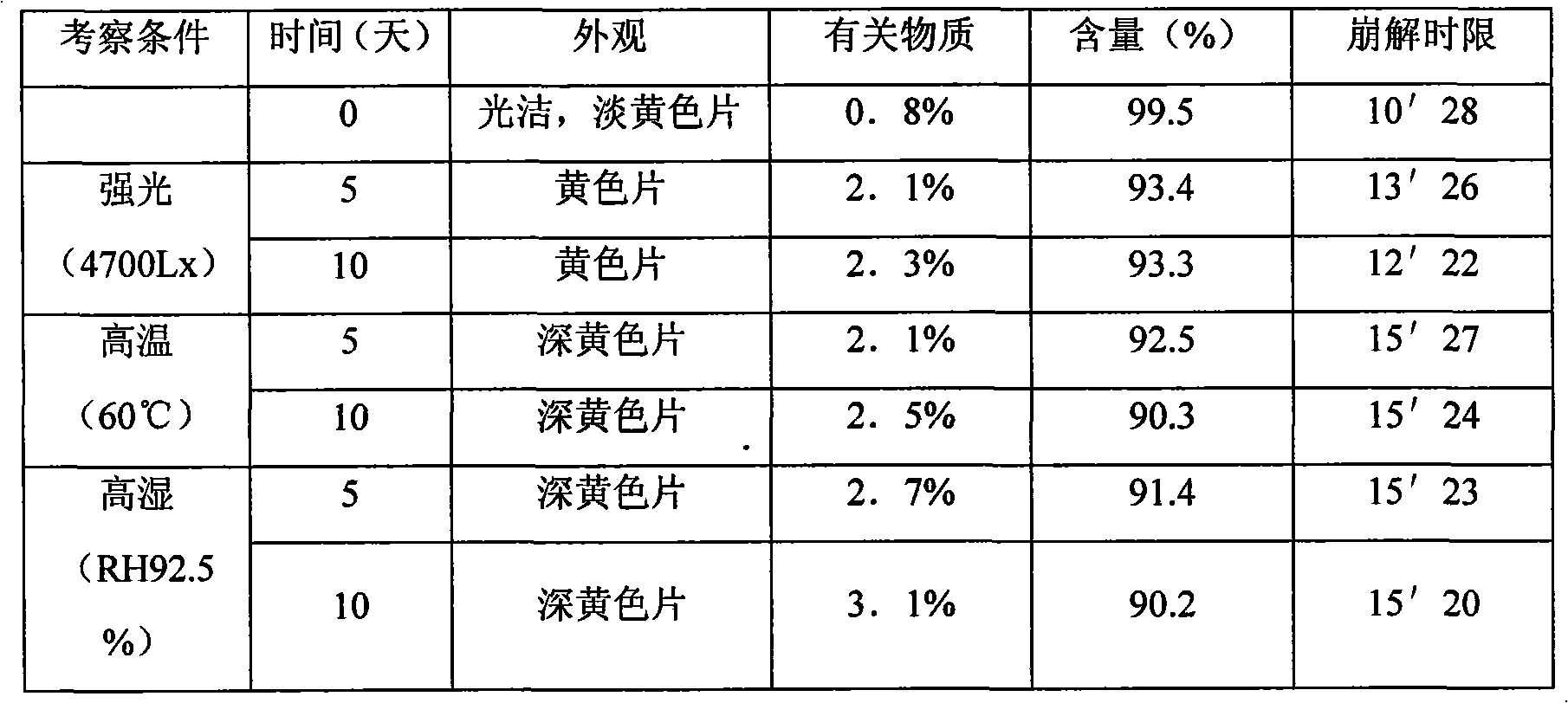
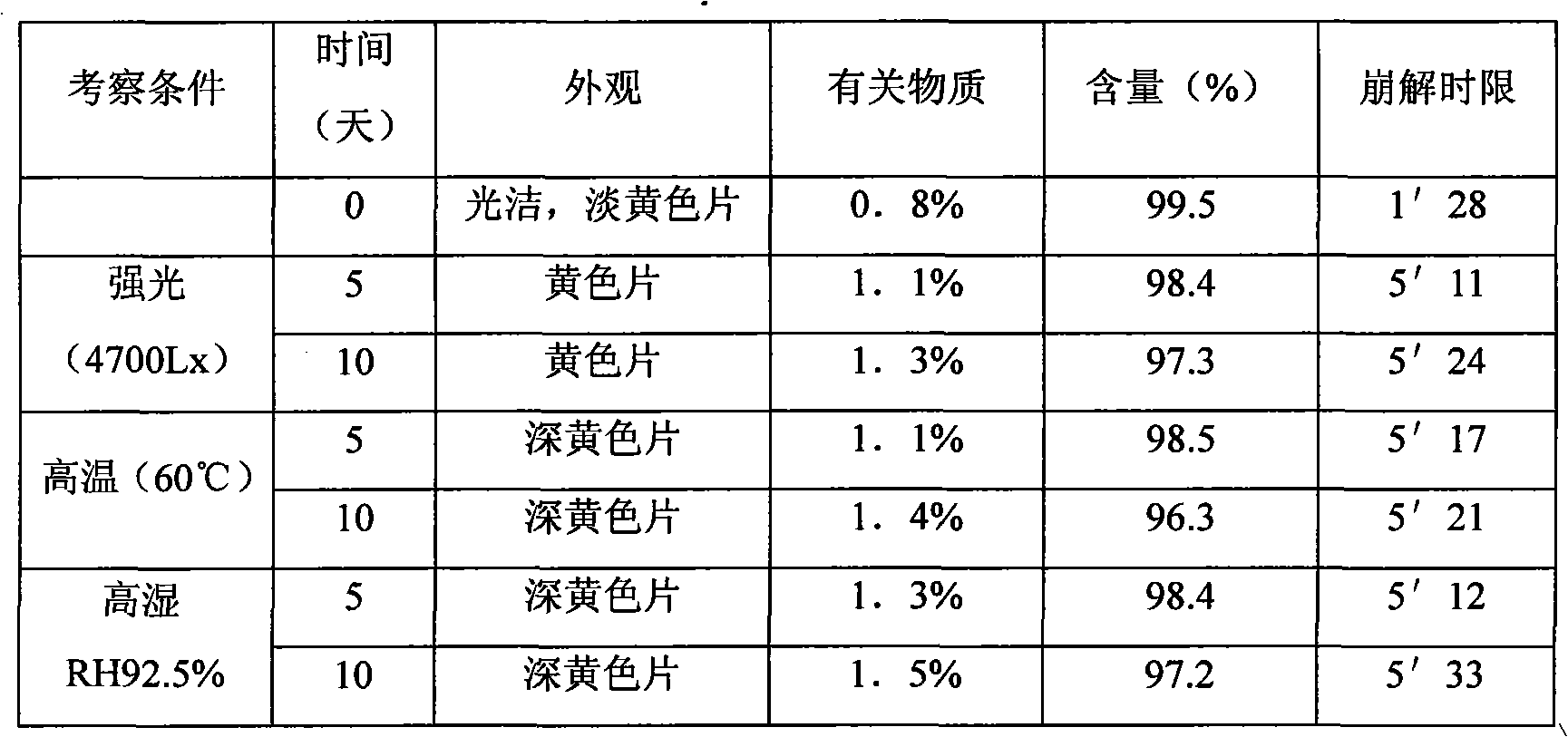
The technology of a composition and caffeic acid is applied in the directions of drug combinations, pharmaceutical formulations, medical preparations of inactive ingredients, etc., which can solve the problems of low bioavailability, unstable products, and large adverse reactions, and achieve rapid drug release, Complete disintegration and complete absorption effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0117] Prepare 1000 caffeic acid tablets whose specification is 0.1 parts by weight / tablet, and the prescription is as follows:
[0118] (1) A particles
[0119] 100 parts by weight of caffeic acid
[0120] PEG6000 50 parts by weight
[0121] Hypromellose 200 parts by weight
[0122] Xylitol ester 10 parts by weight
[0123] 55% ethanol 40 parts by weight
[0124] (2) B particles
[0125] 100 parts by weight of sodium carboxymethyl starch
[0126] Starch 300 parts by weight
[0127] Xylitol ester 5 parts by weight
[0128] 55% ethanol 100 parts by weight
[0129] (3) plus
[0130] 5 parts by weight of magnesium stearate
[0131] Croscarmellose sodium 8 parts by weight
[0132] The preparation method of above-mentioned tablet is as follows:
[0133] 【Operation process】
[0134] 1. Melt the specified amount of caffeic acid and PEG6000A at about 135°C, then fully mix with the specified amount of hydroxypropylmethylcellulose and sodium carboxymethyl starch, then add xyl...
Embodiment 2
[0138] Prepare 1000 caffeic acid tablets whose specification is 0.1 parts by weight / tablet, and the prescription is as follows:
[0139] (1) A particles
[0140] 100 parts by weight of caffeic acid
[0141] PEG6000 150 parts by weight
[0142] Hypromellose 250 parts by weight
[0143] Xylitol ester 15 parts by weight
[0144] 55% ethanol 150 parts by weight
[0145] Appropriate amount
[0146] (2) B particles
[0147] 200 parts by weight of sodium carboxymethyl starch
[0148] Starch 300 parts by weight
[0149] Xylitol ester 7 parts by weight
[0150] 55% ethanol 175 parts by weight
[0151] (3) plus
[0152] 5 parts by weight of magnesium stearate
[0153] 12 parts by weight of croscarmellose sodium
[0154] The preparation method of above-mentioned tablet is as follows:
[0155] 【Operation process】
[0156] 1. Melt the specified amount of caffeic acid and PEG6000A at about 145°C, then fully mix with the specified amount of hydroxypropylmethylcellulose and sodi...
Embodiment 3
[0160] Prepare 1000 caffeic acid tablets whose specification is 0.1 parts by weight / tablet, and the prescription is as follows:
[0161] (1) A particles
[0162] 100 parts by weight of caffeic acid
[0163] PEG6000 45 parts by weight
[0164] Hypromellose 205 parts by weight
[0165] Xylitol ester 11 parts by weight
[0166] 55% ethanol 120 parts by weight
[0167] (2) B particles
[0168] 98 parts by weight of sodium carboxymethyl starch
[0169] Starch 295 parts by weight
[0170] Xylitol ester 4 parts by weight
[0171] 55% ethanol 120 parts by weight
[0172] (3) plus
[0173] 6 parts by weight of magnesium stearate
[0174] Croscarmellose sodium 9 parts by weight
[0175] The preparation method of above-mentioned tablet is as follows:
[0176] 【Operation process】
[0177] 1. Melt the specified amount of caffeic acid and PEG6000A at about 135°C, then fully mix with the specified amount of hydroxypropylmethylcellulose and sodium carboxymethyl starch, then add x...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com