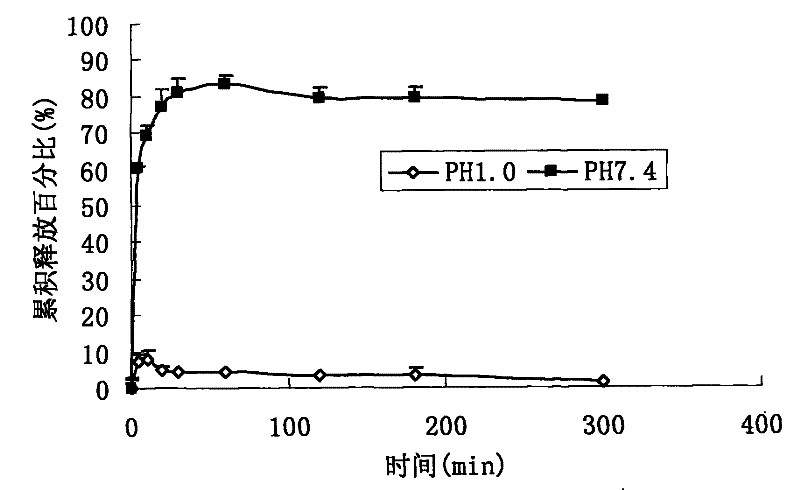
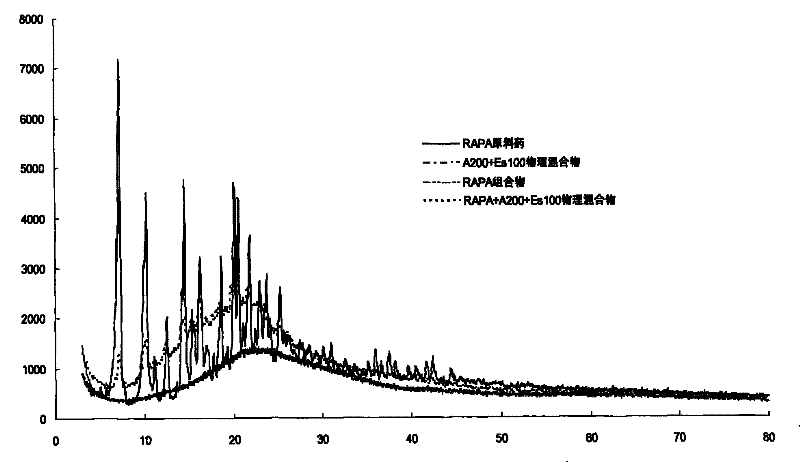
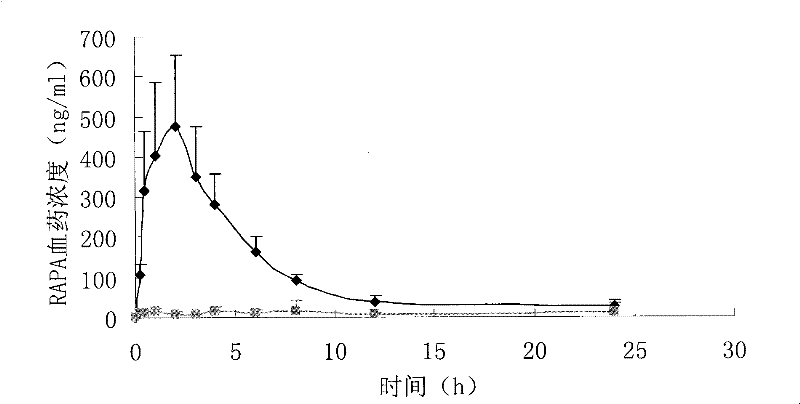
A kind of high bioavailability rapamycin composition and preparation method
A technology of rapamycin and availability, applied in the field of high bioavailability rapamycin composition and preparation, can solve the safety problems of polymer materials and surfactants to be evaluated, and the long-term stability of nanoparticles to be evaluated Solve the problems of complex industrialization and preparation methods, and achieve the effects of increasing bioadhesion, facilitating absorption, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1: Preparation of rapamycin composition
[0049] Weigh 1g of Eudragit S100, fully disperse and dissolve in an appropriate amount of absolute ethanol to form a uniform solution, weigh 0.2g of rapamycin, and dissolve in the above solution; take 1.0g of Aerosil 200 and mix with the above solution to make the liquid Fully adsorbed; the container containing the above mixture was placed in a vacuum desiccator, and the solvent was removed under reduced pressure to form a white blocky solid. Take it out, smash it, sieve it, and get it.
Embodiment 2
[0050] Embodiment 2: Preparation of rapamycin composition
[0051] Weigh 0.9g Eudragit S100, fully disperse and dissolve in absolute ethanol to form a homogeneous solution, weigh 0.3g rapamycin, and dissolve it in the solution; take 0.9g Aerosil 300 powder without mixing with the above solution, make The liquid was fully absorbed; the container containing the mixture was placed in a vacuum desiccator, and the solvent was removed under reduced pressure to form a white blocky solid. Take it out, smash it, sieve it, and get it.
Embodiment 3
[0052] Embodiment 3: Preparation of rapamycin composition
[0053] Weigh 140mg Eudragit S100, fully disperse and dissolve it in absolute ethanol to form a homogeneous solution, weigh 20mg rapamycin and dissolve it in the solution; take 120mg Cab-O-Sil H5 and mix with the above solution to make the liquid Fully adsorbed; the container containing the mixture was placed in a vacuum desiccator, and the solvent was removed under reduced pressure to form a white blocky solid. Take it out, smash it, sieve it, and get it.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com