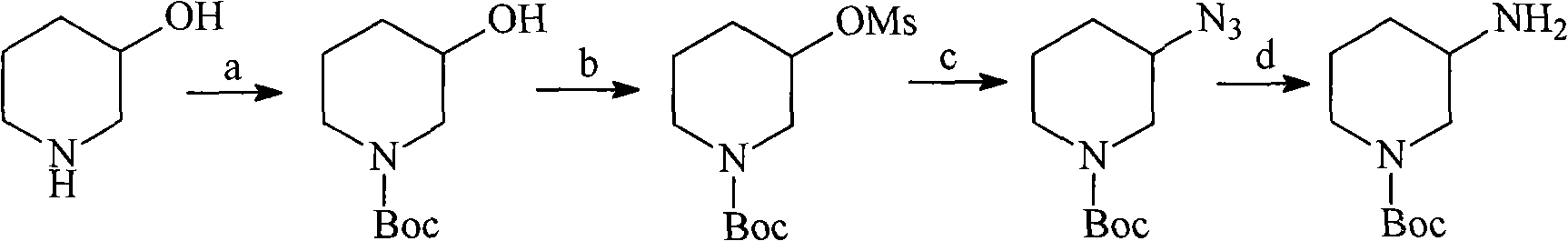
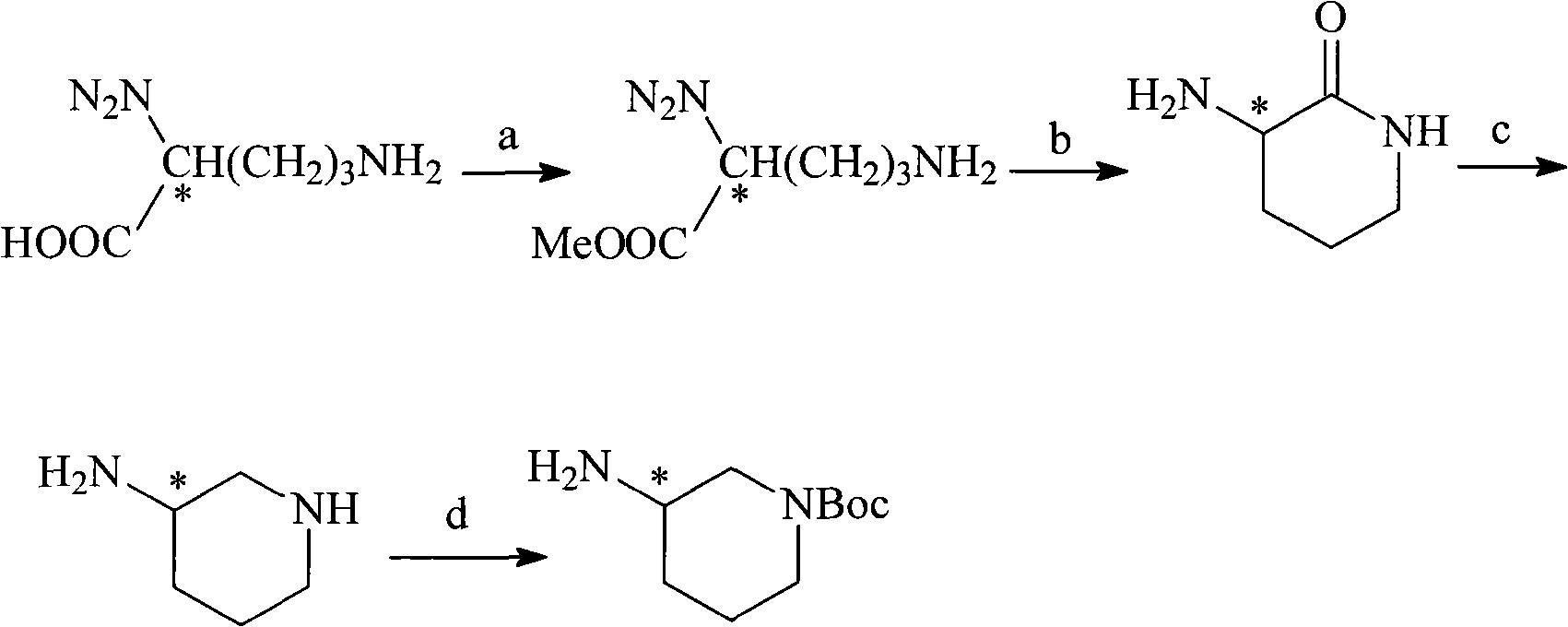
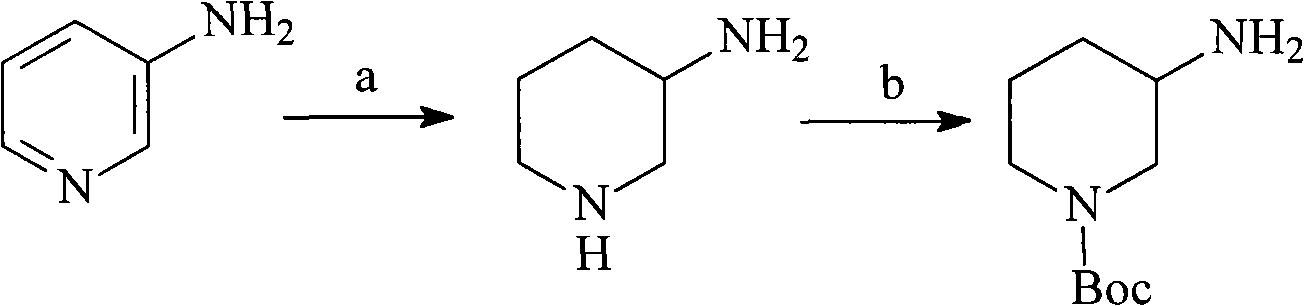
N-Boc-3-aminopiperidine and synthesizing method of optical isomer thereof
A synthetic method, the technology of aminopiperidine, applied in the direction of organic chemical methods, chemical instruments and methods, asymmetric synthesis, etc., can solve the problems of difficult complete hydrogenation, difficult post-processing, difficult industrialization, etc., and achieve low production cost and easy industrialization Large-scale production and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: the synthesis of ethyl N-Boc-3-piperidinecarboxylate (3)
[0032] Dissolve ethyl 3-piperidinecarboxylate (2) (31.4g, 0.2mol) and triethylamine (55.8mL, 0.4mol) in dichloromethane (150mL), cool to below 0°C, add di-tert-dicarbonate dropwise A solution of butyl ester (52.4 g, 0.24 mol) in dichloromethane (100 mL) was controlled at a temperature between 0 and 10°C. After the dropwise addition, wash with saturated potassium carbonate solution (3×50mL), 5% HCl (3×50mL) and saturated brine (3×50mL) successively, dry the organic phase over anhydrous sodium sulfate for 6h, filter and evaporate to dryness under reduced pressure The solvent was used to obtain ethyl N-Boc-3-piperidinecarboxylate (3), 49.3 g, 95%.
Embodiment 2
[0033] Embodiment 2: the synthesis of N-Boc-3-piperidine carboxamide (4)
[0034] Ethyl N-Boc-3-piperidinecarboxylate (3) (41.1g, 0.16mol), 28% ammonia water was dissolved in 1,4-dioxane (300mL), sealed, and the external temperature was set at 85-90 ℃, heat preservation reaction for 1.5h, after the reaction was completed, the solvent was evaporated to dryness under reduced pressure, recrystallized from petroleum ether (340mL), and dried to obtain N-Boc-3-piperidinecarboxamide (4), 35g, 96%.
Embodiment 3
[0035] Embodiment 3: the synthesis of N-Boc-3-aminopiperidine (1)
[0036] Cool NaOH (18g, 0.45mol) and 10% sodium hypochlorite solution (30mL, 0.3mol) to below 0°C, add N-Boc-3-piperidinecarboxamide (4) (34.2g, 0.15mol) in one go, and stir After 30 min, the ice bath was removed. Heat up to 70°C and keep warm for 45 minutes. Cool to room temperature, extract with ether (3×50 mL), combine the organic phases, wash with saturated brine (3×50 mL), and dry over anhydrous sodium sulfate for 6 h. The solvent was evaporated to dryness under reduced pressure to obtain N-Boc-3-aminopiperidine (1), 26.7 g, 89%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com