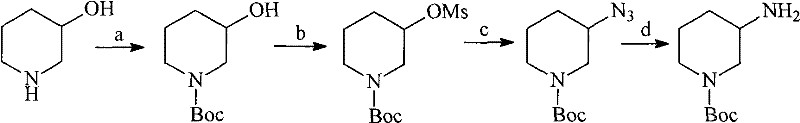
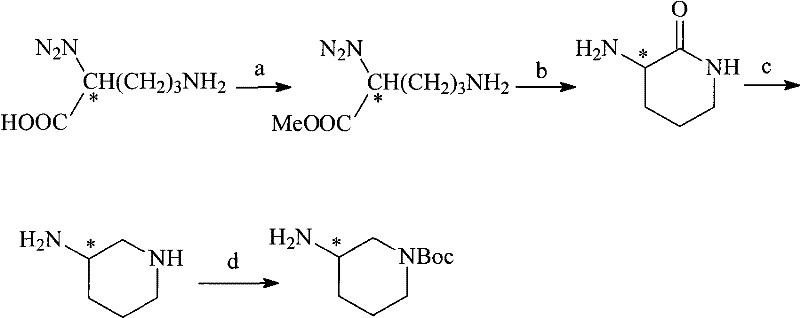
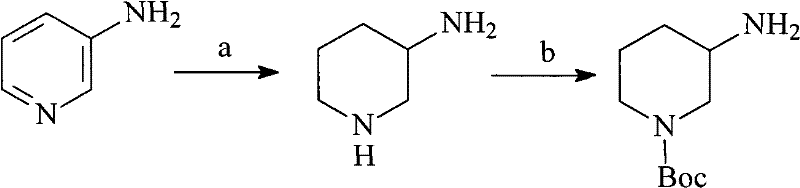
N-Boc-3-aminopiperidine and synthesizing method of optical isomer thereof
A technology of n-boc-3- and -n-boc-3- is applied in the synthesis field of intermediate N-Boc-3-aminopiperidine and optical isomers thereof, and can solve the problems that hydrogenation is not easy to complete and after-treatment Difficulty, difficult to industrialize and other problems, to achieve the effect of low production cost, easy industrialized scale production, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: the synthesis of ethyl N-Boc-3-piperidinecarboxylate (3)
[0032] Dissolve ethyl 3-piperidinecarboxylate (2) (31.4g, 0.2mol) and triethylamine (55.8mL, 0.4mol) in dichloromethane (150mL), cool to below 0°C, add di-tert-dicarbonate dropwise A solution of butyl ester (52.4 g, 0.24 mol) in dichloromethane (100 mL) was controlled at a temperature between 0 and 10°C. After the dropwise addition, wash with saturated potassium carbonate solution (3×50mL), 5% HCl (3×50mL) and saturated brine (3×50mL) successively, dry the organic phase over anhydrous sodium sulfate for 6h, filter and evaporate to dryness under reduced pressure The solvent was used to obtain ethyl N-Boc-3-piperidinecarboxylate (3), 49.3 g, 95%.
Embodiment 2
[0033] Embodiment 2: the synthesis of N-Boc-3-piperidine carboxamide (4)
[0034] Ethyl N-Boc-3-piperidinecarboxylate (3) (41.1g, 0.16mol), 28% ammonia water was dissolved in 1,4-dioxane (300mL), sealed, and the external temperature was set at 85-90 ℃, heat preservation reaction for 1.5h, after the reaction was completed, the solvent was evaporated to dryness under reduced pressure, recrystallized from petroleum ether (340mL), and dried to obtain N-Boc-3-piperidinecarboxamide (4), 35g, 96%.
Embodiment 3
[0035] Embodiment 3: the synthesis of N-Boc-3-aminopiperidine (1)
[0036] Cool NaOH (18g, 0.45mol) and 10% sodium hypochlorite solution (30mL, 0.3mol) to below 0°C, add N-Boc-3-piperidinecarboxamide (4) (34.2g, 0.15mol) in one go, and stir After 30 min, the ice bath was removed. Heat up to 70°C and keep warm for 45 minutes. Cool to room temperature, extract with ether (3×50 mL), combine the organic phases, wash with saturated brine (3×50 mL), and dry over anhydrous sodium sulfate for 6 h. The solvent was evaporated to dryness under reduced pressure to obtain N-Boc-3-aminopiperidine (1), 26.7 g, 89%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com