Reagent for extracting RNA or DNA virus in body fluid
A virus and reagent technology, applied in the field of biochemistry, can solve the problems of cumbersome steps, low efficiency, low extraction efficiency, etc., and achieve the effects of reducing protein impurities, reducing co-precipitation, and improving utilization.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0021] The reagent composition of the present invention: 5.4 mol of guanidine isothiocyanate, 2 mol of guanidine hydrochloride, 25 mmol of sodium citrate, 20 ml of β-mercaptoethanol, 7 g of sodium lauryl sarcosine, 2 mol of urea, 64 mg of glycogen, and the rest is water.
[0022] The preparation of reagent of the present invention: get guanidine isothiocyanate, guanidine hydrochloride, sodium citrate 2, β-mercaptoethanol, sodium lauryl sarcosinate, urea and glycogen mix, dissolve with the water that DEPC is processed and Quantify to 1L, and then filter with a 0.45μm filter.
[0023] Extraction of viral RNA:
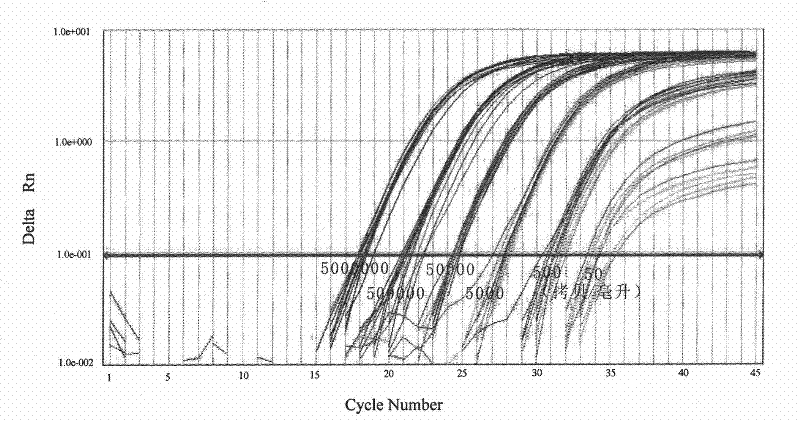
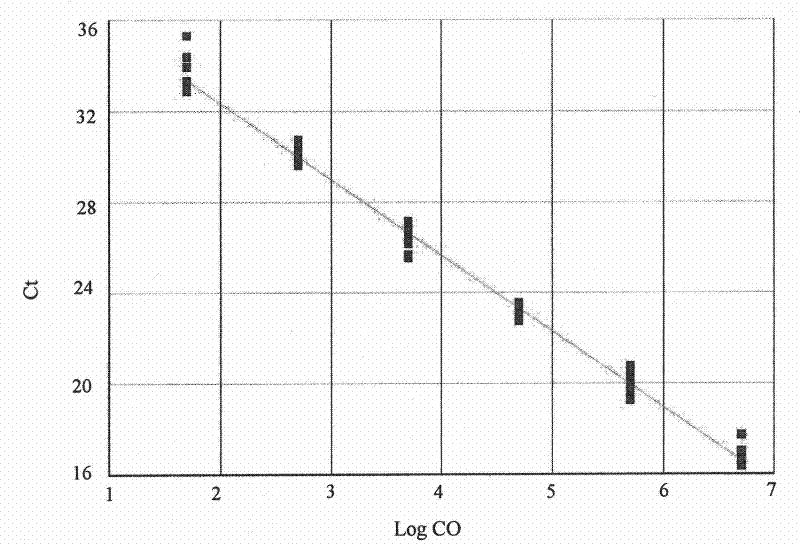
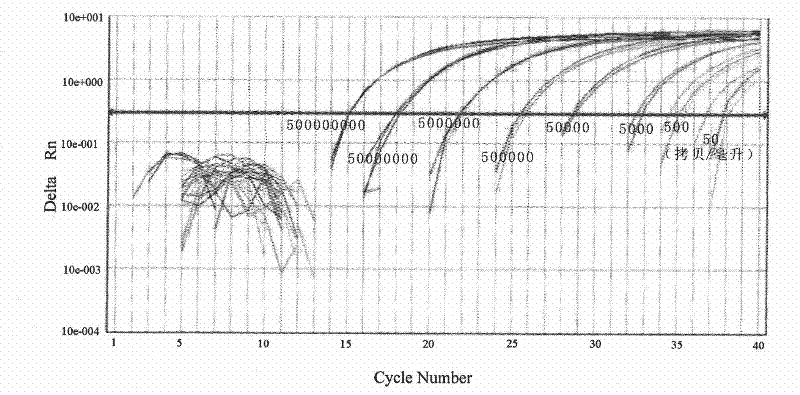
[0024] (1) Materials: Dilute the SIV virus standard product (international general SIV virus particle standard product, gifted by Professor Lu Wei of Paris Fifth University, France) with monkey plasma to 6 concentrations, respectively: 0.5×10 7 copies / ml, 0.5×10 6 copies / ml, 0.5×10 5 copies / ml, 0.5×10 4 copies / ml, 0.5×10 3 copies / ml, 0.5×10 2 copies / ml, 12 parallel ...
example 2
[0050] The reagent composition of the present invention: 5.4 mol of guanidine isothiocyanate, 2 mol of guanidine hydrochloride, 25 mmol of sodium citrate, 20 ml of β-mercaptoethanol, 7 g of sodium lauryl sarcosine, 2 mol of urea, 64 mg of glycogen, and the rest is water.
[0051] Preparation of reagent of the present invention: get guanidine isothiocyanate, guanidine hydrochloride, sodium citrate, β-mercaptoethanol, sodium lauryl sarcosinate, urea and glycogen mix, dissolve and quantify with DEPC-treated water to 1 L, and then filtered through a 0.45 μm filter.
[0052] Experimental materials: Serum samples from patients with a quantitative HBVDNA copy number above 1e9 detected clinically in the hospital.
[0053] Dilute the serum samples of HBVDNA patients with newborn bovine serum for cell culture, and the dilutions are: 5e8 copies / ml, 5e7 copies / ml, 5e6 copies / ml, 5e5 copies / ml, 5e4 copies / ml, 5e3 copies / ml, 5e2 copies / ml, 5e1 copies / ml, 3 duplicate holes are set for each...
example 3
[0066] The reagent composition of the present invention: 4 mol of guanidine isothiocyanate, 1 mol of guanidine hydrochloride, 20 mmol of sodium citrate, 10 ml of β-mercaptoethanol, 5 g of sodium lauryl sarcosine, 1.5 mol of urea, 60 mg of glycogen, and the balance is water.
[0067] Preparation of reagent of the present invention: get guanidine isothiocyanate, guanidine hydrochloride, sodium citrate, β-mercaptoethanol, sodium lauryl sarcosinate, urea and glycogen mix, dissolve and quantify with DEPC-treated water to 1 L, and then filtered through a 0.45 μm filter.
[0068] Experimental materials: Serum samples from patients with a quantitative HBVDNA copy number above 1e9 detected clinically in the hospital.
[0069] Dilute the serum samples of HBVDNA patients with newborn bovine serum for cell culture, and the dilutions are: 5e8 copies / ml, 5e7 copies / ml, 5e6 copies / ml, 5e5 copies / ml, 5e4 copies / ml, 5e3 copies / ml, 5e2 copies / ml, 5e1 copies / ml, 3 duplicate holes are set for e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com