Method for preparing mozavaptan
A technology of formula and halogenated alkanes, which is applied in the field of preparation of mozavaptan, can solve the problems of low yield and achieve the effects of high reaction yield, simple post-treatment and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
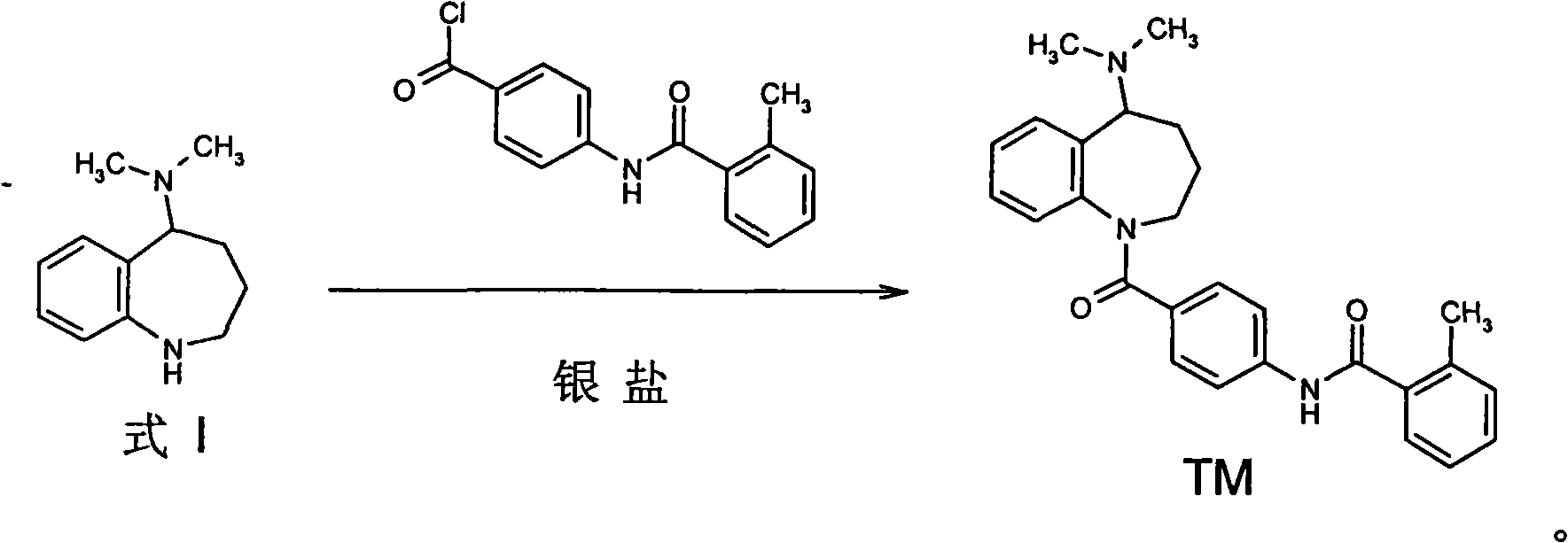
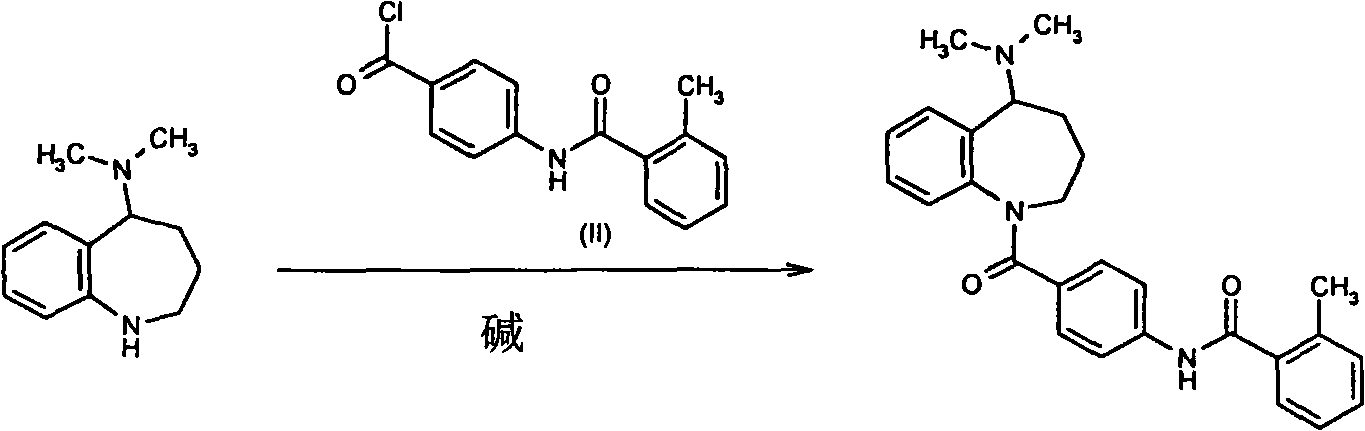
[0029] 1.9 g of 5-dimethylamino-2,3,4,5-tetrahydro-1H-benzazepine (Formula 1) was dissolved with 3 g of 4-(o-methylbenzamido)-benzoyl chloride In 20 ml of dichloromethane, 5 ml of tetrahydrofuran solution in which 2.3 g of silver trifluoromethanesulfonate was dissolved was added dropwise at 0 to -5°C, and after the addition was complete, the reaction was carried out at room temperature for 30 minutes. Add 10 ml of saturated sodium bicarbonate solution and stir for 10 minutes, filter, wash the filter cake with a small amount of dichloromethane, combine the organic phases, wash twice with 10 ml of water, and dry overnight with 5 g of anhydrous sodium sulfate. After recovering the solvent, the solid was recrystallized from ethanol to obtain 3.4 g of mozavaptan, with a yield of 79.6%.
Embodiment 2
[0031] 1.9 g of 5-dimethylamino-2,3,4,5-tetrahydro-1H-benzazepine (Formula 1) was dissolved with 3 g of 4-(o-methylbenzamido)-benzoyl chloride In 20 ml of 1,2-dichloroethane, 10 ml of dioxane solution in which 2.1 g of silver trifluoroacetate was dissolved was added dropwise at 0 to -5°C. After the addition was complete, the reaction was carried out at room temperature for 30 minutes. Add 10 ml of saturated potassium bicarbonate solution and stir for 10 minutes, filter, wash the filter cake with a small amount of 1,2-dichloroethane, combine the organic phases, wash twice with 10 ml of water, and dry overnight with 5 g of anhydrous sodium sulfate. After recovering the solvent, the solid was recrystallized from ethanol to obtain 3.3 g of mozavaptan, with a yield of 77.3%.
Embodiment 3
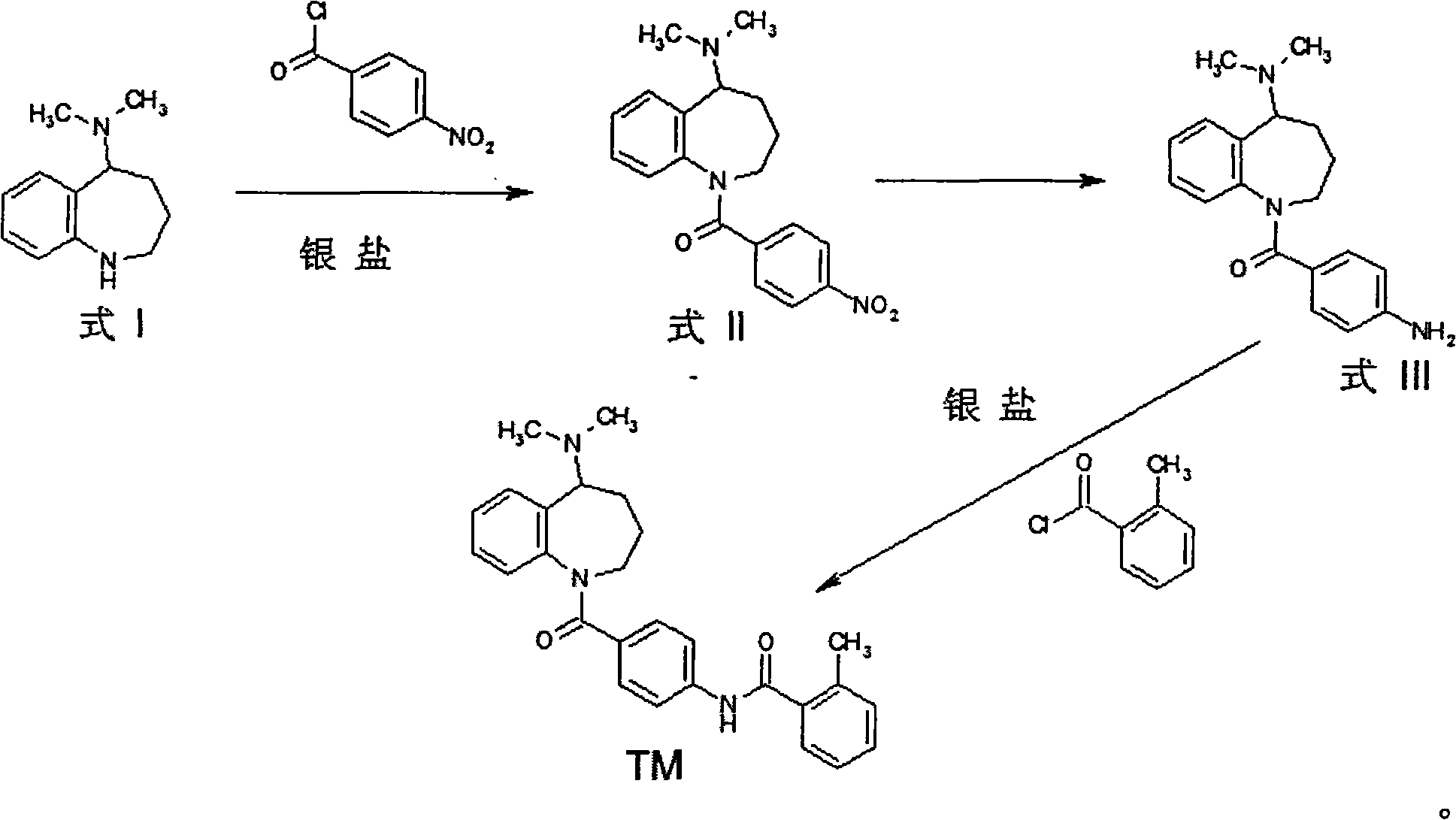
[0033] Dissolve 1.9 g of 5-dimethylamino-2,3,4,5-tetrahydro-1H-benzazepine (Formula 1) and 1.6 g of p-nitrobenzoyl chloride in 20 ml of 1,2-dichloro In ethane, at 0~-5°C, 5 ml of tetrahydrofuran solution in which 2.3 g of silver trifluoromethanesulfonate was dissolved was added dropwise, and after the addition was completed, the reaction was carried out at room temperature for 30 minutes. Add 10 ml of saturated sodium bicarbonate solution and stir for 10 minutes, filter, wash the filter cake with a small amount of dichloromethane, combine the organic phases, wash twice with 10 ml of water, and dry overnight with 5 g of anhydrous sodium sulfate. After recovering the solvent, the solid was recrystallized from ethanol to obtain 2.7 g of formula II, which was reduced in 30 ml of absolute ethanol with 0.2 g of 5% palladium carbon hydrogenation under normal pressure. After recovering the solvent, dissolve it in 20ml of dichloromethane, add 1.3 grams of o-toluyl chloride, and add dro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com