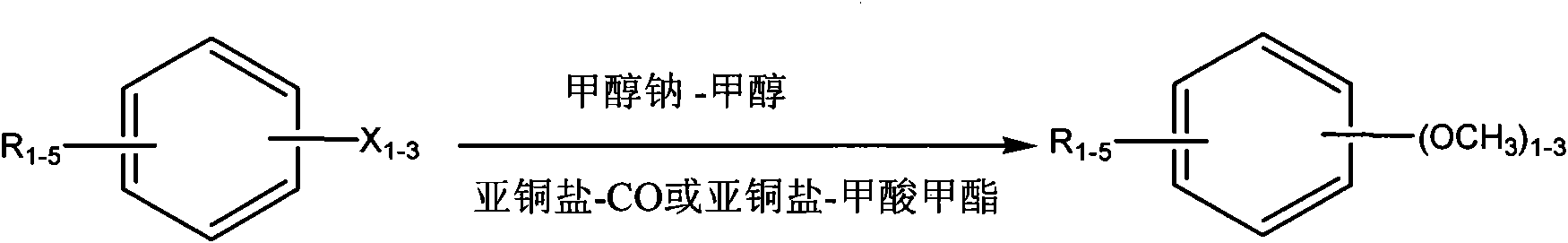
Preparation method of aromatic methyl ether compound
A technology for aromatic compounds and aromatic methyl ethers, applied in ether preparation, organic chemistry, etc., can solve problems such as adverse impacts on production costs, unfavorable product quality, impacts, etc., to reduce the possibility of debromination reduction reactions and reduce production Cost, effect of improving product quality and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
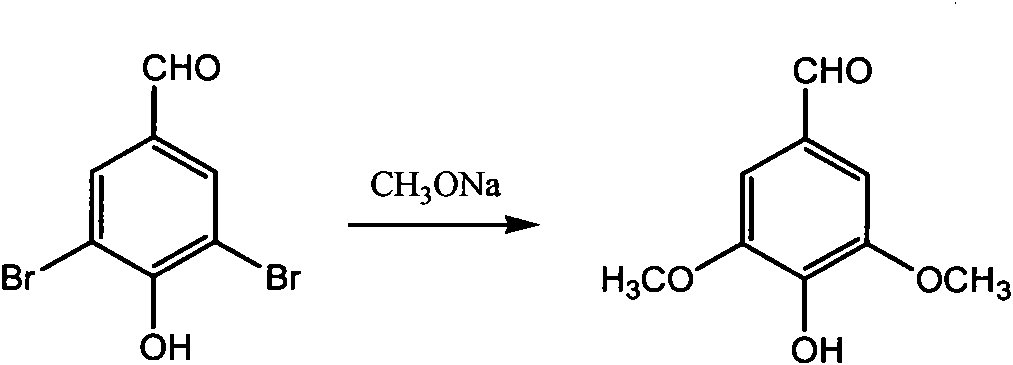
[0026] Embodiment 1 synthesizes syringaldehyde
[0027] See attached figure 2 137.8g, 29% sodium methoxide methanol solution (0.74mol), 56.0g of 4-hydroxyl-3,5-dibromobenzaldehyde (0.20mol), 2.8g of chlorinated methoxide were added successively in a 500mL autoclave Copper (0.028 mol). Seal the autoclave, feed about 2.8 g of carbon monoxide (0.10 mol), stir, heat up to 120° C. and keep it warm for 3 hours, stop heating and cool to room temperature. Carefully open the autoclave, transfer the reaction mixture to a flask, distill and recover methanol, add 250 mL of water, and raise the temperature to 90°C to dissolve the sodium phenolate salt of 4-hydroxy-3,5-dimethoxybenzaldehyde. The catalyst was removed by hot filtration, the filtrate was cooled to room temperature, acidified with dilute hydrochloric acid to pH=5.0-6.0, and a large amount of white solid was precipitated. Filter and dry the filter cake to obtain 35.2 g of white syringaldehyde (4-hydroxy-3,5-dimethoxybenzalde...
Embodiment 2
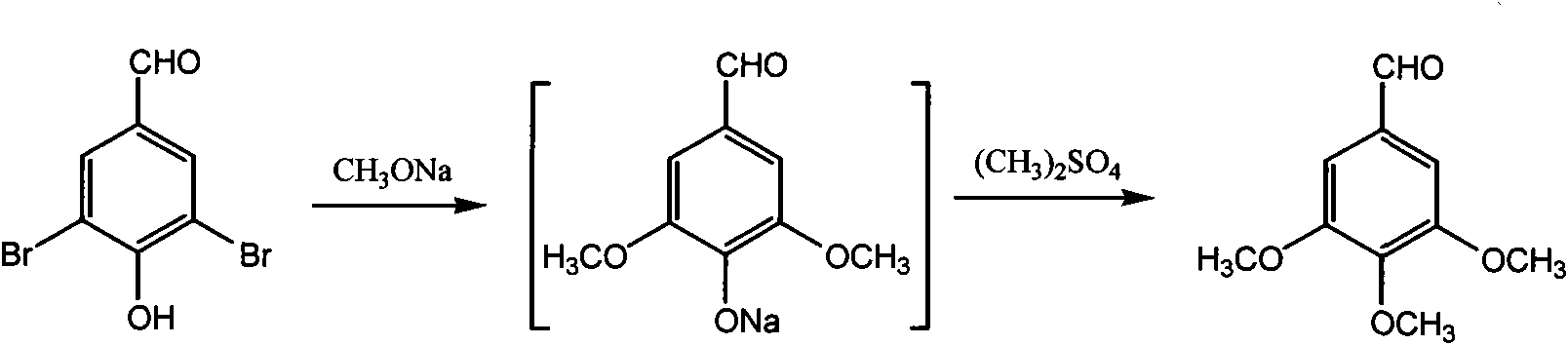
[0030] Synthesis of 3,4,5-trimethoxybenzaldehyde in embodiment 2
[0031] See attached image 3 137.8g, 29% sodium methoxide methanol solution (0.74mol), 56.0g of 4-hydroxyl-3,5-dibromobenzaldehyde (0.20mol), 2.8g of chlorinated methoxide were added successively in a 500mL autoclave Copper (0.028 mol), 5.0 g of methyl formate (0.083 mol). Seal the autoclave, stir, heat up to 120°C and keep it warm for 3 hours, stop heating and cool to room temperature. Carefully open the autoclave, transfer the reaction mixture to a flask, distill and recover methanol, add 100mL of water, raise the temperature to 90°C to dissolve the eugenalphenol sodium salt, and freeze to 10°C to crystallize the phenol sodium salt. Filter to separate the filter cake of eugenol sodium salt. Add 250 mL of water to the filter cake of syringaldehyde phenol sodium salt, and heat up to 90° C. to dissolve the phenol sodium salt. Heat filtration removes catalyzer, the sodium phenate mother liquor of filtering is...
Embodiment 3
[0034] Example 3 Synthesis of 3,4,5-trimethoxytoluene
[0035] See attached Figure 4100.5 g, 29% sodium methoxide methanol solution (0.54 mol), 56.0 g of 4-methoxy-3,5-dibromotoluene (0.20 mol), 2.8 g of chlorinated Cuprous (0.028 mol), 5.0 g of methyl formate (0.083 mol). Seal the autoclave, stir, heat up to 120°C and keep it warm for 3 hours, stop heating and cool to room temperature. Carefully open the autoclave, transfer the reaction mixture to a flask, distill off the recovered methanol and distill the residue under reduced pressure, collect the fraction with a boiling point (bp) of 102-104°C / 450Pa, and the product solidifies to give colorless 3, 4, 5 - 34.7 g of trimethoxytoluene solid, melting point (mp) 33-35° C., yield 95.3%. HPLC test purity ≥ 99.0%.
[0036] 1 H NMR (500MHz, CDCl 3 ): 2.32 (s, 3H, CH 3 ), 3.82 (s, 3H, OCH 3 ), 3.85(s, 6H, OCH 3 ), 6.40 (s, 2H, ArH).
[0037] EI-MS m / z: 182 (M + , 85), 167(100), 139(37), 124(20), 109(17).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com