Method for removing residual palladium in crude product of faropenem sodium prepared by palladium catalysis method
A technology catalyzed by faropenem sodium and palladium, which is applied in chemical instruments and methods, physical/chemical process catalysts, organic compounds/hydrides/coordination complex catalysts, etc., and can solve the problems of high industrial production costs and low product yields, etc. problems, to achieve the effect of simple operation, solving technical problems, and good adsorption effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
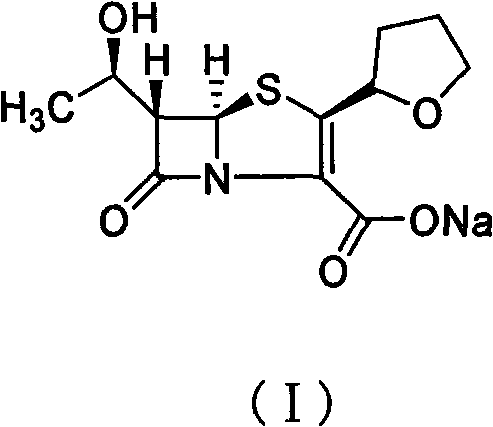
[0024] A palladium catalyzed preparation of crude faropenem sodium (I):
[0025] Put sodium isooctanoate (4.33g, 0.026mol) and deionized water (3.4mL) in the reaction flask and stir until dissolved, then add (1′R, 2″R, 5R, 6S)-6-[(1′ -Hydroxyethyl)-2"-tetrahydrofuryl]penicillene-3-carboxylic acid allyl ester (8.5g, 0.026mol) and ethyl acetate (34mL) solution, and replace the air in the reaction flask with nitrogen and quickly add Tetrakis(triphenylphosphine)palladium (0.3g, 0.26mmol) and triphenylphosphine (0.34g, 1.3mmol), after stirring at 25°C for 5 hours, cooling to 0°C and stirring for 0.5 hours, filtering, filter cake After washing with ethyl acetate, the crude faropenem sodium (I) was obtained. The crude product was detected by ICP emission spectrometer and the palladium content was 68 ppm.
[0026] B. Removal of residual palladium:
[0027] The crude faropenem sodium (I) (palladium content 0.578mmol) was prepared into a saturated aqueous solution (24.5mL) with deionized wa...
Embodiment 2
[0029] A palladium catalyzed preparation of crude faropenem sodium (I):
[0030] Place sodium bicarbonate (5.2g, 61.4mmol) and deionized water (8mL) in a reaction flask, stir and add 5,5-dimethylcyclohexanedione (5.2g, 37.2mmol) in batches, and wait until the addition is complete. Then continue to stir until the reaction solution is clear, and then add (1′R,2″R,5R,6S)-6-[(1′-hydroxyethyl)-2″-tetrahydrofuryl]penem-3-carboxylic acid After a solution of allyl ester (20g, 61.4mmol) and ethyl acetate (80mL), and replacing the air in the reaction flask with nitrogen, bis(triphenylphosphine)palladium dichloride (1.0g, 1.43mmol) was quickly added to After stirring for 5 hours at 25°C, then cooling to 0°C and stirring for 0.5 hours, filtering, and washing the filter cake with ethyl acetate, it is the crude faropenem sodium (I). The crude product was detected by ICP emission spectrometer and the palladium content was 104ppm.
[0031] B. Removal of residual palladium:
[0032] The crude faro...
Embodiment 3
[0034] A palladium catalyzed preparation of crude faropenem sodium (I):
[0035]Place sodium bicarbonate (5.2g, 61.4mmol) and deionized water (8mL) in a reaction flask, stir and add 5,5-dimethylcyclohexanedione (5.2g, 37.2mmol) in batches, and wait until the addition is complete. Then continue to stir until the reaction solution is clear; then add (1′R,2″R,5R,6S)-6-[(1′-hydroxyethyl)-2″-tetrahydrofuryl]penem-3-carboxylic acid After a solution of allyl ester (20g, 61.4mmol) and acetone (80mL), and replacing the air in the reaction flask with nitrogen, quickly add palladium dichloride (0.25g, 1.43mmol) and triphenylphosphine (0.674g, 2.57mmol) ). After stirring at 25°C for 5 hours, then cooling to 0°C and stirring for 0.5 hours, filtering, and washing the filter cake with ethyl acetate, it is the crude faropenem sodium (I). This crude product is detected by ICP emission spectrometer. The content of palladium is It is 200ppm.
[0036] B. Removal of residual palladium:
[0037] The cr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com