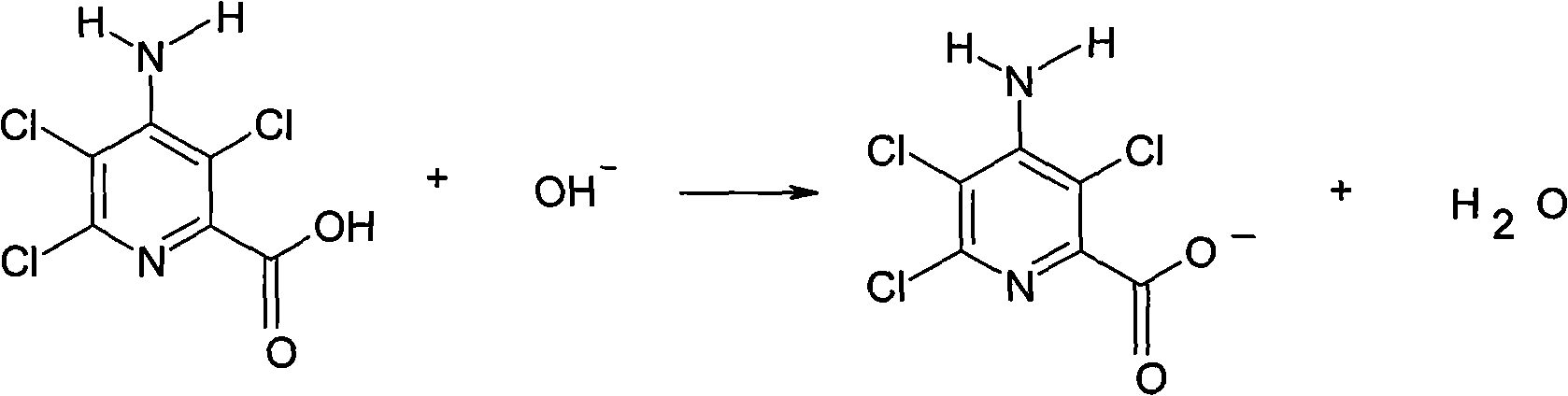
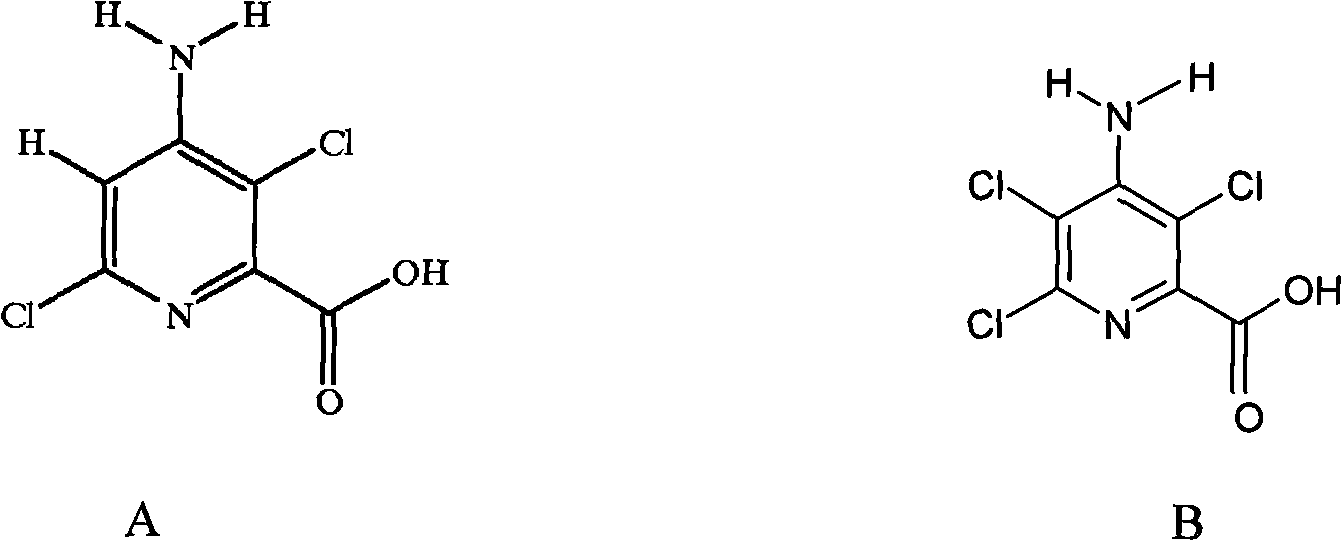Method for electrolytic synthesis of 4-amino-3,6-dichloropyridine-2-carboxylic acid
A technology of dichloropyridine and electrolytic synthesis, which is applied in the direction of electrolytic process, electrolytic components, electrolytic organic production, etc., can solve the problems of difficult control of the reaction, expensive raw materials used, unfriendly environment, etc., and achieve less investment and reduced side effects of industrial production. Reaction, the effect of no three wastes pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Prepare 450ml electrolyte solution, which contains 95.5% 4-amino-3,5,6-trichloropyridine-2-carboxylic acid 33g, containing 1.5% sodium hydroxide, and the electrolyte solution is under magnetic stirring, and the constant potential electrolysis power supply is turned on, and the control The potential of the saturated calomel reference electrode is between -1100mv~-1350mv, after adding 32% sodium hydroxide solution for 24 hours, sampling and analysis, when the content of 4-amino-3,5,6-trichloropyridine-2-carboxylic acid When it is less than 0.6%, stop the power supply and stop the reaction; the electrolyte is neutralized with 36% hydrochloric acid, concentrated in vacuum below 65°C to 180ml, cooled to normal temperature, filtered, washed 3 times with 20ml water (total water volume 60ml), 80°C After drying, 23.17 g of the product was obtained. Through high-performance liquid chromatography analysis, the content was 94%, the melting point was 185-189° C., and the yield was 81...
Embodiment 2
[0035] According to embodiment 1, replace sodium hydroxide with potassium hydroxide, alkalinity is controlled at 1.0%~1.5%, and reaction temperature is controlled at 20 ℃, and the total consuming time of reaction completion is 22 hours, and aftertreatment method is the same as above, obtains product 25.1g, The content is 96.5%, the melting point is 184-187°C, and the yield is 90%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com