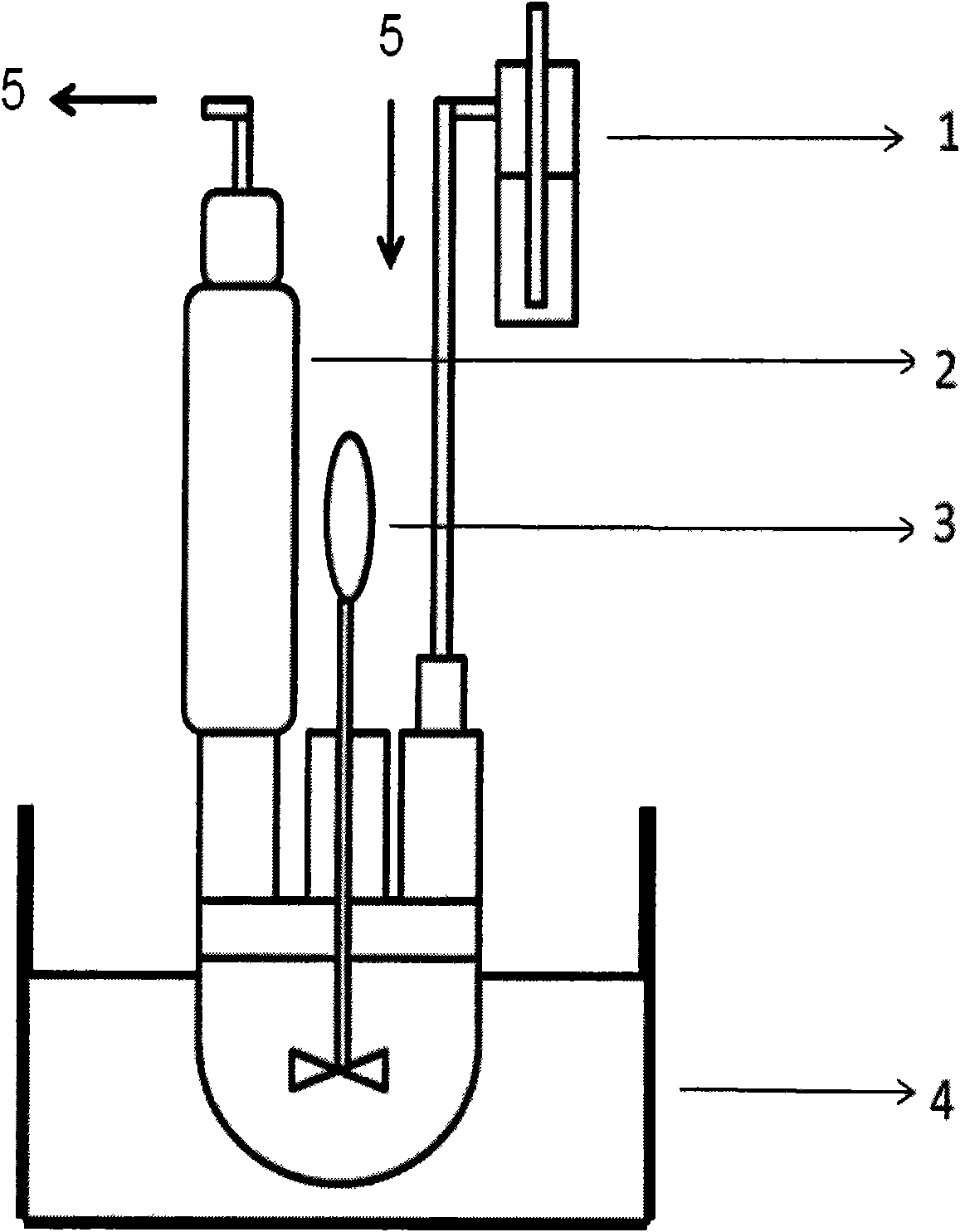
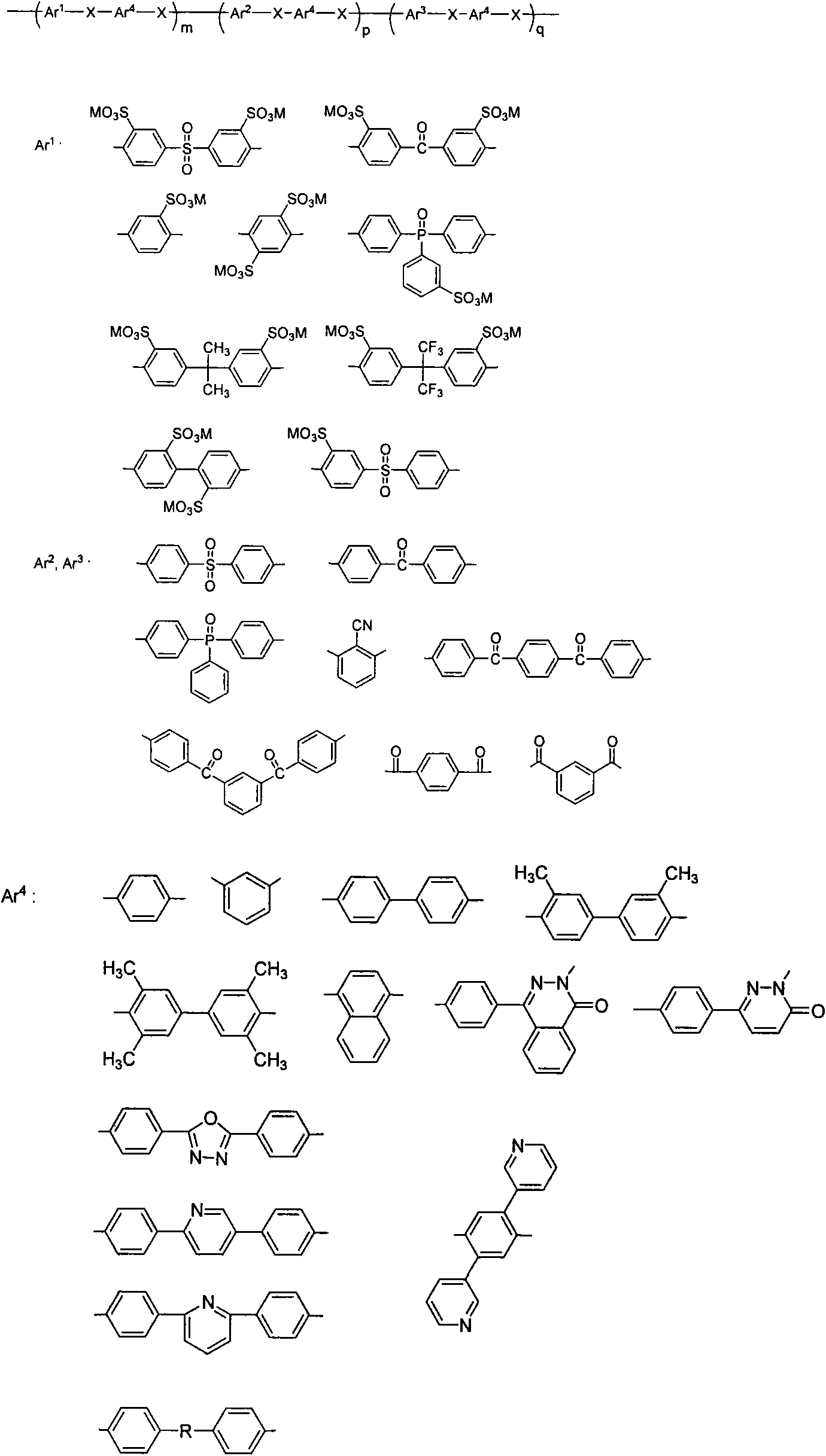Method for preparing aromatic nucleophilic substitution polymer under anhydrous condition
A nucleophilic substitution and polymer technology, applied in the field of preparing aromatic nucleophilic substitution polymers under novel anhydrous conditions, can solve the problems of complicated recycling process, large reaction liquid volume, long reaction time, etc., and reduce energy consumption , simplified process, high molecular weight effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
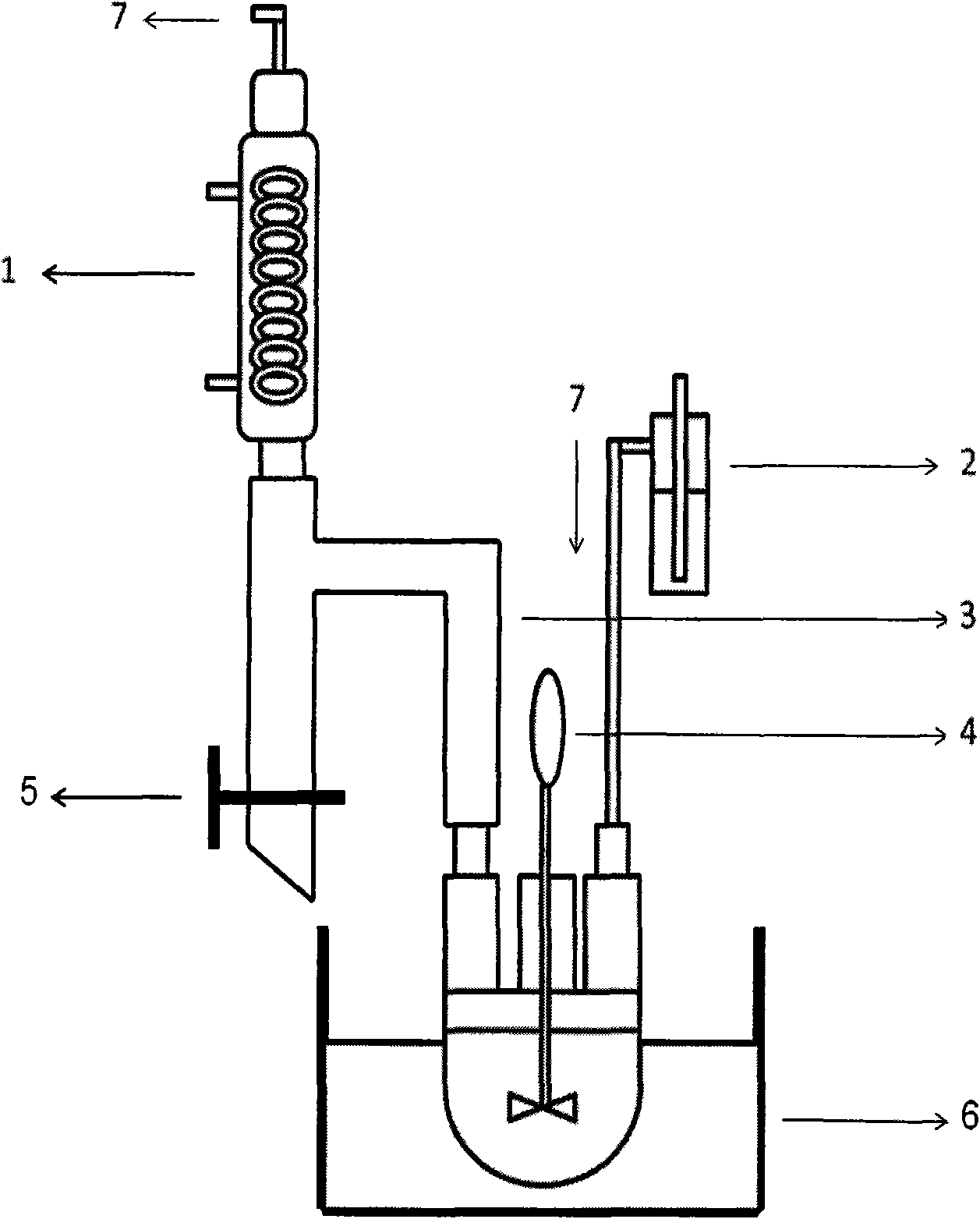
[0045] according to figure 2 In the shown reaction device, 3,3'-sodium disulfonate-4,4'-dichlorodiphenylsulfone (SDCDPS, 17.1938g, 35mmol), 4,4'-dichlorodiphenylsulfone (DCDPS, 18.6654 g, 65mmol), 4,4'-biphenol (BP, 18.6210g, 100mmol), anhydrous potassium phosphate (K 3 PO 4 , 46.6994g, 220mmol), 272mL N,N-dimethylacetamide (DMAc) were mixed, the reaction temperature was raised to 180°C, and the reaction was carried out for 12 hours. Stop heating and stirring, and cool to room temperature naturally. The reaction solution was slowly poured into 2L of deionized water to obtain a white fibrous polymer, soaked in 4L of deionized water for 8 hours at 80°C, repeated three times, filtered, dried, and then vacuum-dried at 100°C for 24 hours to obtain a shallow Yellow fibrous polymer (sulfonated polyethersulfone compound), 46.8 g, yield: 99%, intrinsic viscosity: 0.98 dL / g.
Embodiment 2
[0047] according to figure 2 In the shown reaction device, 3,3'-sodium disulfonate-4,4'-dichlorodiphenylsulfone (SDCDPS, 17.1938g, 35mmol), 4,4'-dichlorodiphenylsulfone (DCDPS, 18.6654 g, 65mmol), 4,4'-biphenol (BP, 18.6210g, 100mmol), anhydrous potassium phosphate (K 3 PO 4 , 46.6994g, 220mmol), 272mL of N-methylpyrrolidone (NMP) were mixed, the reaction temperature was raised to 190°C, and the reaction was carried out for 12 hours. Stop heating and stirring, and cool to room temperature naturally. The reaction solution was slowly poured into 2L of deionized water to obtain a light yellow fibrous polymer, soaked in 4L of deionized water at 80°C for 8 hours, repeated three times, filtered, dried, and then vacuum-dried at 100°C for 24 hours to obtain Yellow fibrous polymer (sulfonated polyethersulfone compound), 44.2 g, yield: 94%, intrinsic viscosity: 0.91 dL / g.
Embodiment 3
[0049] according to figure 2 In the shown reaction device, 3,3'-sodium disulfonate-4,4'-dichlorodiphenylsulfone (SDCDPS, 17.1938g, 35mmol), 4,4'-dichlorodiphenylsulfone (DCDPS, 18.6654 g, 65mmol), 4,4'-biphenol (BP, 18.6210g, 100mmol), anhydrous potassium carbonate (K 2 CO 3 , 27.6420g, 200mmol), 272mL N,N-dimethylacetamide (DMAc) were mixed, the reaction temperature was raised to 180°C, and the reaction was carried out for 24 hours. Stop heating and stirring, and cool to room temperature naturally. The reaction solution was slowly poured into 2L of deionized water to obtain a white fibrous polymer, soaked in 4L of deionized water for 8 hours at 80°C, repeated three times, filtered, dried, and then vacuum-dried at 100°C for 24 hours to obtain a shallow Yellow fibrous polymer (sulfonated polyethersulfone compound), 46.8 g, yield: 99%, intrinsic viscosity: 1.14 dL / g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com