New immunosuppressive agent extracted from Honghuoma and extraction method and use thereof
An immunosuppressant and weighing technology, which is applied in the field of medical chemical drugs, can solve the problems of limited application of side effects, short drug action cycle, and no ideal effective drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
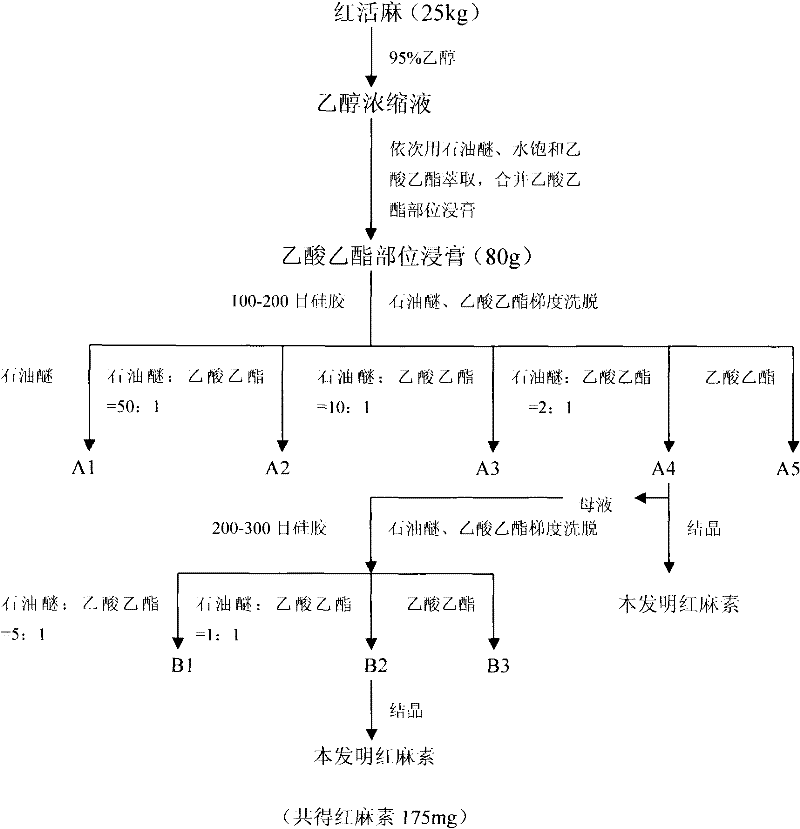
[0085] Example 1. Extraction and separation of ethyl acetate part of red live hemp
[0086] Weigh the washed and air-dried red live hemp, soak it with 95% ethanol for several times (5-8 times) after crushing, soak the solvent every time to cover the medicinal materials, soak for 3-5 days each time, take the soaking liquid and recover it under reduced pressure To obtain the ethanol extract of Honghuoma, suspend the ethanol extract of Honghuoma in water and then extract with petroleum ether, water-saturated ethyl acetate, water-saturated n-butanol and water, respectively, to obtain petroleum ether extract, ethyl acetate extract, Butanol extract and water extract. The red live hemp used in this example comes from Enshi Autonomous Prefecture, Hubei Province, my country.
Embodiment 2
[0087] Example 2. The extraction and separation of novel immunosuppressant erythromycin
[0088] a. Completely dissolve the extract of the ethyl acetate part with an organic solvent and put it on a silica gel column, and use eluents of different polarities prepared by two solvents, petroleum ether and ethyl acetate, to elute sequentially according to the polarity from small to large, The elution order is: petroleum ether, petroleum ether: ethyl acetate = 50:1, petroleum ether: ethyl acetate = 10:1, petroleum ether: ethyl acetate = 2:1 and water-saturated ethyl acetate. Ether: ethyl acetate = 2: 1 Pale yellow needle-like crystals were precipitated in some fractions, and the mother liquor was changed to a 200-300 mesh silica gel column to continue separation. Petroleum ether: ethyl acetate = 5: 1, petroleum ether: ethyl acetate Esters = 1:1 and ethyl acetate were eluted separately, and light yellow needle crystals also precipitated in the fraction of petroleum ether: ethyl aceta...
Embodiment 3
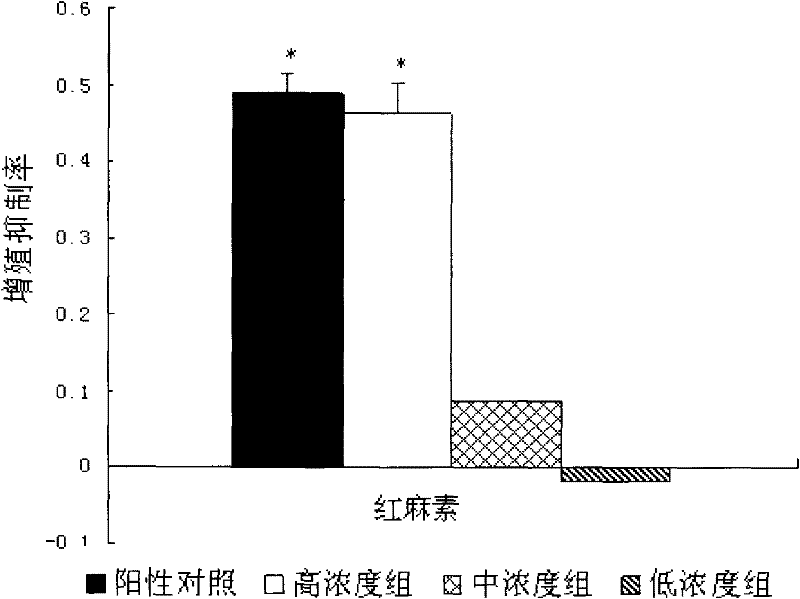
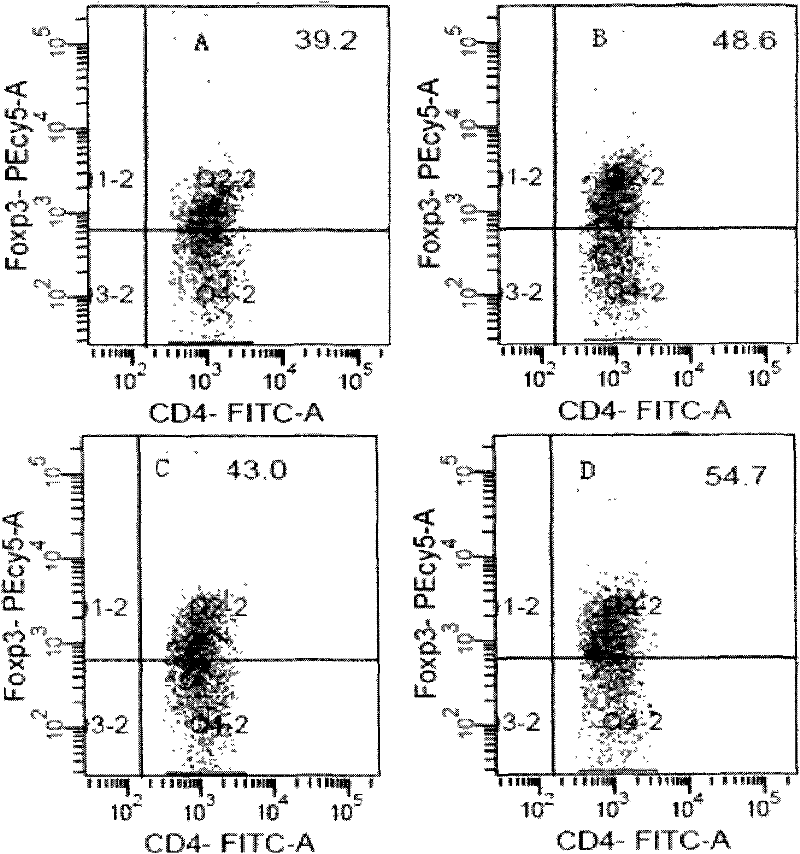
[0095] Embodiment 3 Preparation of different dosage forms of immunosuppressant erythromycin of the present invention
[0096] There is no clear limitation on the dosage form of the immunosuppressant containing erythromycin in the present invention, and various types of oral or parenteral preparations can be made according to actual clinical conditions. In order to meet the needs of clinical treatment of autoimmune diseases, the invention also provides a clinically suitable dosage form for the novel immunosuppressant.
[0097] 1. Separation and purification of kenafin: take the washed and air-dried red hemp, soak it several times (5 to 8 times) with 95% ethanol after crushing, and make the solvent submerge the medicinal material for each soaking, and soak for 3 to 5 times each time. After taking the soaking liquid and recovering it under reduced pressure, the ethanol extract of Honghuoma was obtained. After suspending the ethanol extract of Honghuoma in water, it was extracted ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com