Flavanone derivatives, preparation method and use thereof
A technology for dihydroflavonoids and derivatives, which is applied in the field of dihydroflavonoid derivatives and their preparation, can solve the problems of inability to be widely used, difficult extraction of natural dihydroflavonoids, and difficulty in obtaining them.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
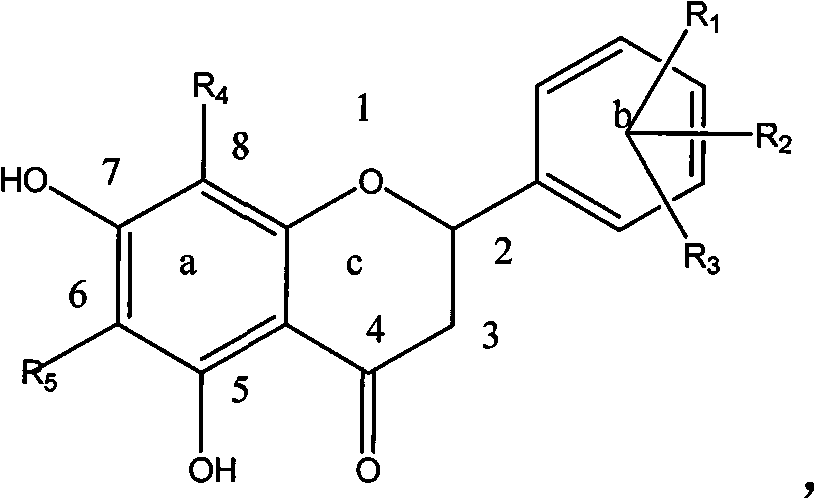
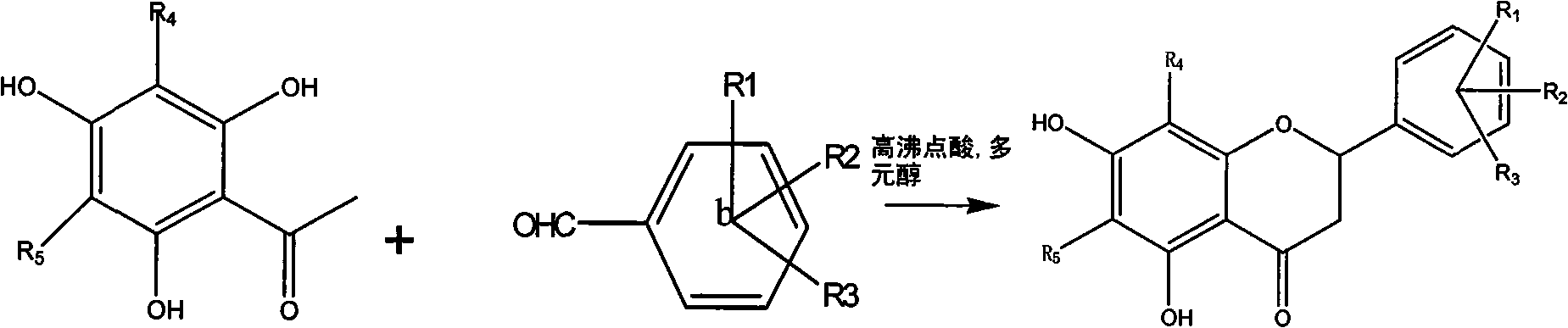
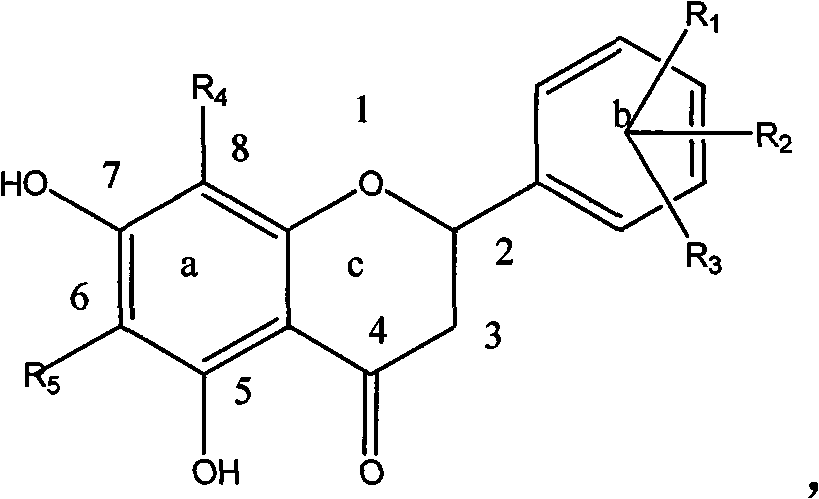
[0054] Example 1: Preparation of Compound 1-a: (6' Hydroxy-5,7 Dihydroxy-6,8-Dimethyldihydroflavone):
[0055] Dissolve 200 mg (0.001mol) of 6-hydroxyl-2,4-hydroxy-3,5-dimethylacetophenone, 125 mg (0.001mol) of 2-hydroxybenzaldehyde, and 61 mg (0.001mol) of boric acid in a small amount of ethylene glycol In alcohol, heat up at 100°C, stir and react for 1 hour, dissolve the reactant with ethanol, and obtain 30 mg of a light green substance through column chromatography, with a yield of 15%, HNMR (500MHz, DMSO), δ7.02 (2H, d , J=8.4Hz, H-e), 6.75 (1H, d, J=8.5Hz, H-d), 6.65 (1H, d, J=8.5Hz, H-d), 5.58 (1H, dd, J=12.3, 2.7Hz, H-c), 3.18 (1H, dd, J=17.0, 12.5Hz, H-b 2 ), 2.86 (1H, dd, J=17.0, 3.0Hz, H-b 1 ), 1.97 (6H, d, J=4.5Hz, H-a) ESI-MS (m / z): [M-1] - =299.
Embodiment 2
[0056] Example 2: Preparation of Compound 1-b: (4' Chloro-5,7 Dihydroxy-6,8-Dimethyldihydroflavone):
[0057] Dissolve 200 mg (0.001mol) of 6-hydroxy-2,4-hydroxy-3,5-dimethylacetophenone, 700 mg (0.005mol) of 4-chlorobenzaldehyde, and 270 mg (0.005mol) of citric acid in a small amount of ethanol , the temperature was raised at 200°C, the reaction was stirred for 5 hours, the reactant was dissolved in ethanol, and 25 mg of a light green substance was obtained by column chromatography, with a yield of 12.5%, HNMR (500MHz, DMSO), δ7.56 (2H, d, J = 8.4Hz, H-e), 7.51 (2H, d, J = 8.5Hz, H-d), 5.58 (1H, dd, J = 12.3, 2.7Hz, H-c), 3.18 (1H, dd, J = 17.0, 12.5Hz , H-b2), 2.86 (1H, dd, J=17.0, 3.0Hz, H-b1), 1.97 (6H, d, J=4.5Hz, H-a) ESI-MS (m / z): [M-1 ]-=317.
Embodiment 3
[0058] Example 3: Compound 1-n: 2-(furan-2-yl)-5,7-dihydroxy-6,8-methyl-chroman-4-one
[0059] 200 mg (0.001mol) of 6-hydroxyl-2,4 hydroxy-3,5 dimethylacetophenone, 0.3ml (0.0015mol) of furfural, and 250 mg (0.004mol) of tartaric acid were dissolved in a small amount of glycerol, and the temperature was raised in 50 DEG C, stirred and reacted for 15 hours, the reactant was dissolved in ethanol, and 35 mg of a light yellow substance was obtained through column chromatography, with a yield of 15%.
[0060] HNMR (500MHz, DMSO) δ7.36 (H, d, J=8.4Hz, H-e), 6.26 (H, d, J=8.5Hz, H-d), 6.19 (H, d, J=8.5Hz, H-d), 5.74 (1H, dd, J=12.3, 2.7Hz, H-c), 3.18 (1H, dd, J=17.0, 12.5Hz, H-b 2 ), 2.85 (1H, dd, J=17.0, 3.0Hz, H-b 1 ), 1.99 (6H, d, J=4.5Hz, H-a) ESI-MS (m / z): [M-1] - =257.
[0061] The following compounds were synthesized according to the above preparation methods.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com