Gimeracil crystal form and preparation method thereof
A technology of crystal form and mass ratio, applied in the field of medicine and chemical industry, can solve the problems of time-consuming and labor-intensive, no research on the conversion between crystal forms, no research on high-purity products, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] The preparation of embodiment 1 type A crystal seed
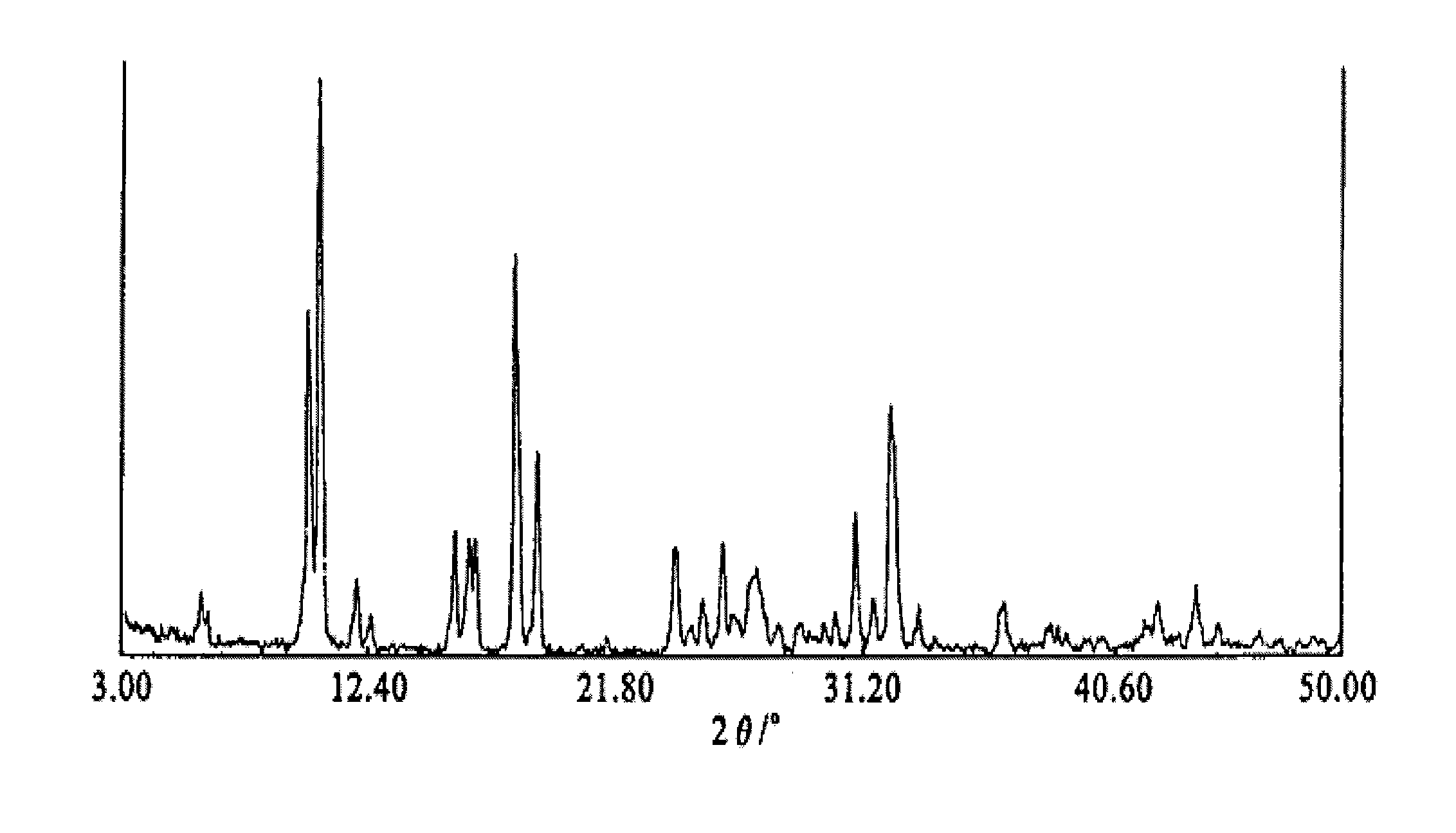
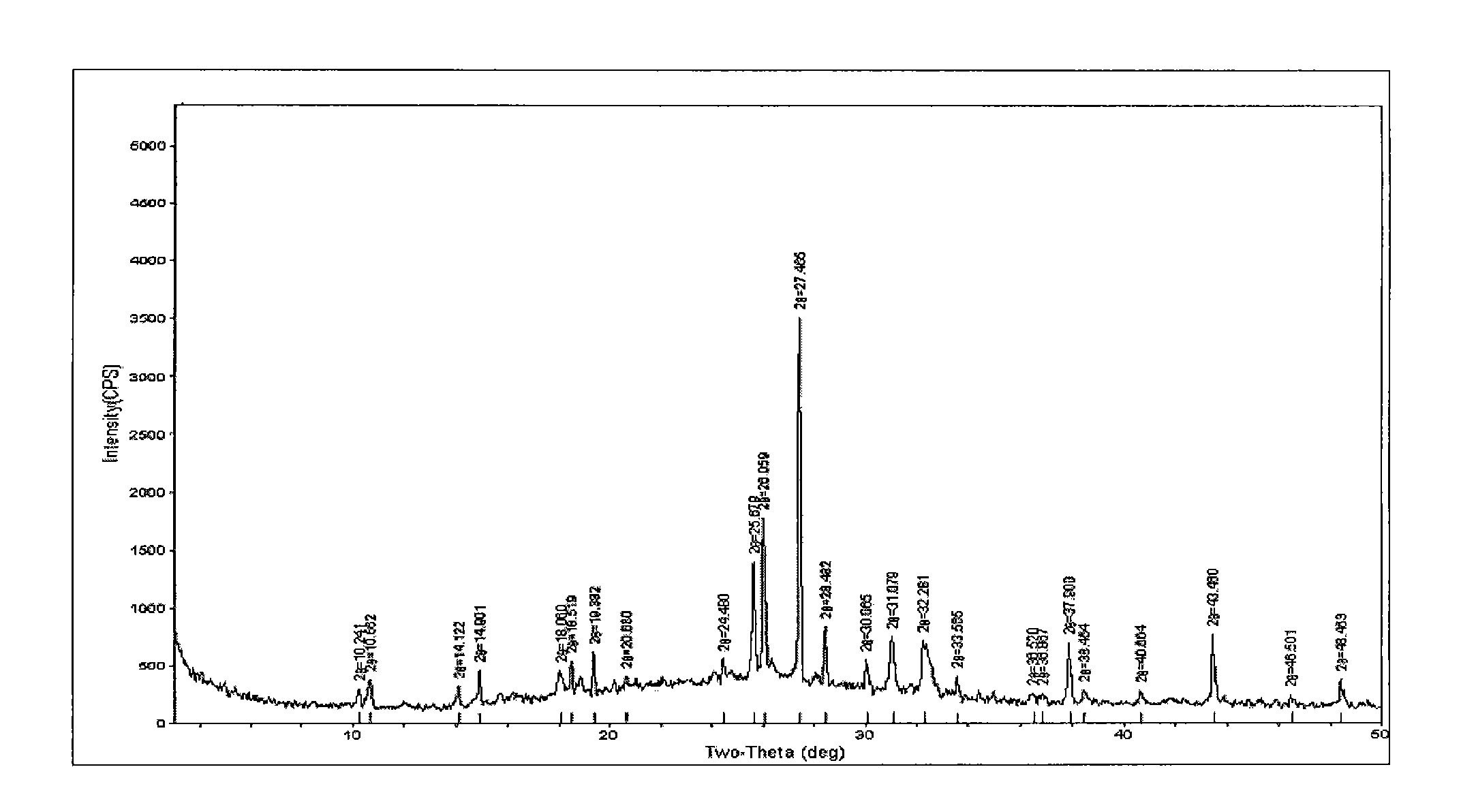
[0083] 10 grams of gimostert heated and dissolved in 500 milliliters of water are synthesized by the method provided in the literature of "The Synthesis of Gymaster" (Chinese Journal of Medicinal Chemistry, 81 [18-1], February 2008, 44-47), Add 1 gram of activated carbon, heat to reflux, vacuum filter while it is hot, let it stand without stirring, cool with ice water to precipitate white needle-shaped crystals, cool to 10°C and filter with suction, wash the filter cake with a small amount of water, drain and filter The cake was dried in an oven at 60°C for 5 hours. Obtained 9.4 g of dry Gimostel white needle-like crystals—type A crystals, HPLC 99.82%, yield 99.4%. Powder X-ray Diffraction see figure 2 , melting point 277.11-277.61°C ( Figure 6 ), see the infrared spectrum Figure 11 .
Embodiment 2
[0084] Example 2 Preparation of Gimerost Type A Crystals in Ethanol Solution
[0085] Heat and dissolve 10 grams of P-type crystal Gimostel in 350 ml of 94% ethanol-water solution, let it stand without stirring, cool with ice water to precipitate crystals, and filter the precipitated crystals after cooling to 10°C, and use a small amount of ethanol in the same proportion Wash with aqueous solution, and dry the filter cake in an oven at 60°C for 5 hours. Obtained dry gimoster type A crystals, the content and yield are shown in the table below. Gimostel uses 90%, 80%, 70%, 60%, 50%, 30%, 20% ethanol-water solution and water respectively to prepare Gimostil A-type crystals according to the above process conditions, and the content and yield Rates are shown in the table below. Crystal powder X-ray diffraction see figure ( figure 2 ).
[0086]
[0087]
Embodiment 3
[0088] Example 3 Preparation of Gimerost P-type Crystals in Ethanol Solution
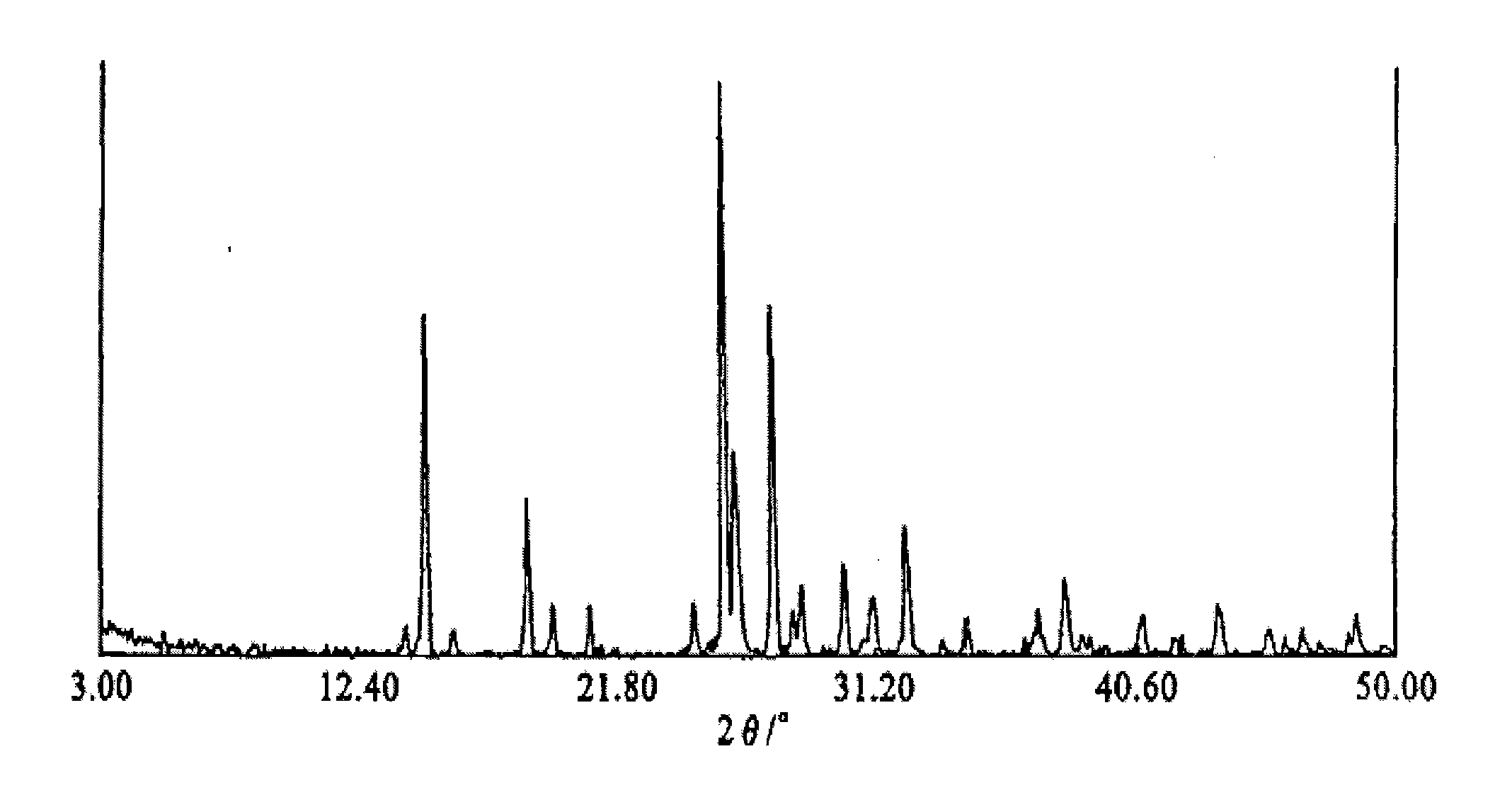
[0089]Heat and dissolve 10 g of crystal form A gimostel in 350 ml of 94% ethanol-water solution, add P-type crystal seed crystals and stir vigorously, the precipitated crystals are cooled to 10°C, then suction filtered, and washed with a small amount of ethanol water solution in the same proportion , The filter cake was dried in an oven at 60°C for 5 hours. The dry Gymast P-type crystals were obtained, and the contents and yields are shown in the table below. Gimostel uses 90%, 80%, 70%, 60%, 50%, 40%, 30% ethanol-water solution and water respectively to prepare Gimostel P-type crystals according to the above process conditions, and the content and yield Rates are shown in the table below. Crystal powder X-ray diffraction see Figure 4 .
[0090]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com