Method for separating and comprehensively utilizing nitrobenzene chlorides
A technology of nitrobenzene dichloride and chloride is applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., and can solve the problems of waste of resources, pollution, difficulty in separation of nitrobenzene chloride, etc. Bring economic benefits and solve the effect of environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
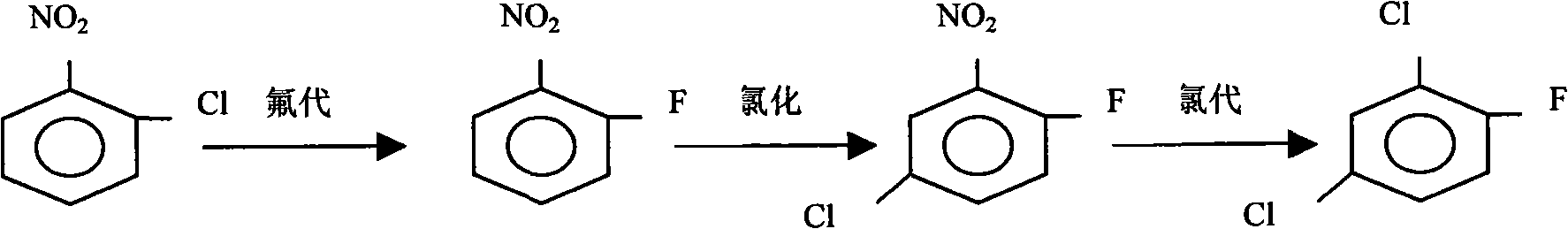
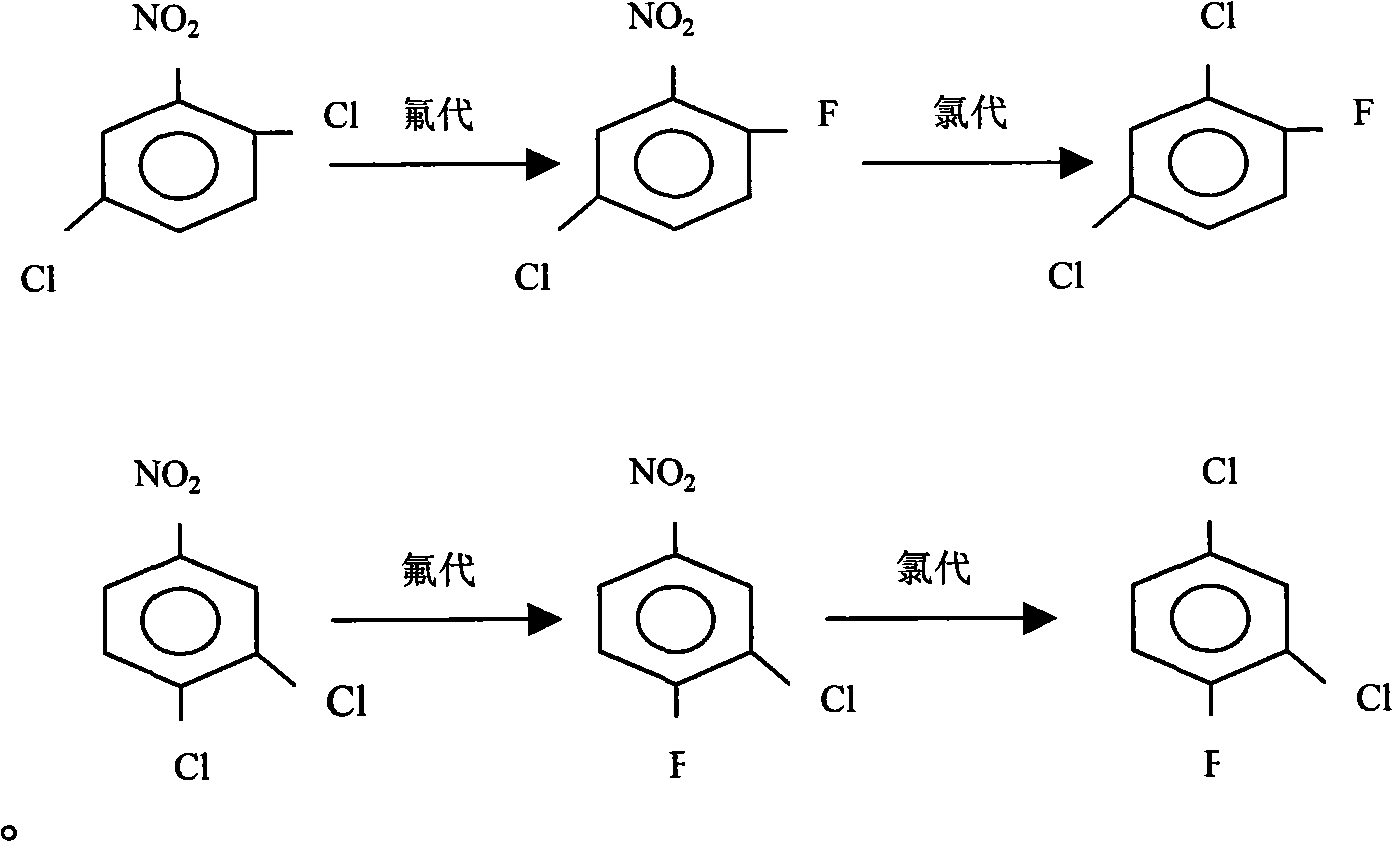
[0027] Put nitrobenzene monochloride, DMF solvent, and anhydrous potassium fluoride into a stirred reactor, use hexadecyltrimethylammonium bromide as a phase transfer catalyst, and control the temperature at 160-180°C for heat preservation reaction , after the reaction is completed, steam distillation is carried out to separate the organic layer, the water layer is incorporated into the next steam distillation, and the organic layer is rectified to obtain the finished products o-, p-nitrofluorobenzene and m-nitrochlorobenzene.
example 2
[0029] (1), using the o- and p-nitrofluorobenzenes obtained in Example 1 as raw materials, using ferric chloride as a catalyst, put them into a chlorination kettle, feed chlorine gas, and control the reaction temperature at 80-95 ° C, and detect with GC Chlorination depth, remove residual chlorine and hydrogen chloride gas after the reaction, and obtain 2-fluoro-5-chloronitrobenzene and 3-chloro-4-fluoronitrobenzene through rectification;
[0030] (2), raise the temperature of the kettle to 200-220 ° C, feed chlorine gas, and continuously add the mixture of 2-fluoro-5-chloronitrobenzene and 3-chloro-4-fluoronitrobenzene that was prepared in the previous step, drop The acceleration is equivalent to the evaporation rate, and the bottom liquid in the reactor is recycled, and more than 80% of the crude product of 2,4-dichlorofluorobenzene is evaporated, and the crude product is rectified, and the fraction at 170-172 ° C is collected to obtain 2 with a purity of more than 99.5%. 4-...
example 3
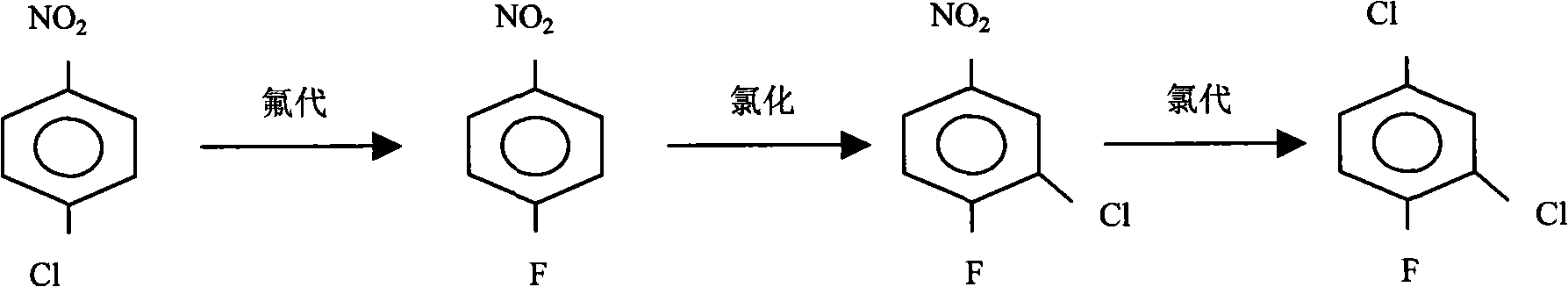
[0032] (1), nitrobenzene dichloride is dropped in the reaction kettle, with DMF as solvent, Potassium fluoride is fluorinating agent, is the mixture that is 90% with quaternary ammonium salt content and 10% quaternary phosphonium salt content as catalyst, React at 175-185°C to generate 3-chloro-4-fluoronitrobenzene, 2-fluoro-5-chloronitrobenzene and potassium chloride, remove potassium chloride by filtration, and then obtain 3-chloro-4-fluoronitrobenzene through rectification and 2-fluoro-5 chloronitrobenzene mixture;
[0033] (2), raise the temperature of the kettle to 200-220 ° C, feed chlorine gas, and continuously add the mixture of 2-fluoro-5-chloronitrobenzene and 3-chloro-4-fluoronitrobenzene that was prepared in the previous step, drop The acceleration is equivalent to the evaporation rate, and the bottom liquid in the reactor is recycled, and more than 80% of the crude product of 2,4-dichlorofluorobenzene is evaporated, and the crude product is rectified, and the frac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com