Combined attenuated live vaccine for measles, mumps and encephalitis B and preparation method thereof
A technology of attenuated live vaccines and Japanese encephalitis, applied in antiviral agents, pharmaceutical formulas, medical preparations containing active ingredients, etc. It is suitable for large-scale production, can not meet the requirements of unit price seedlings, etc., and achieves the effect of simple preparation process and guaranteed stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
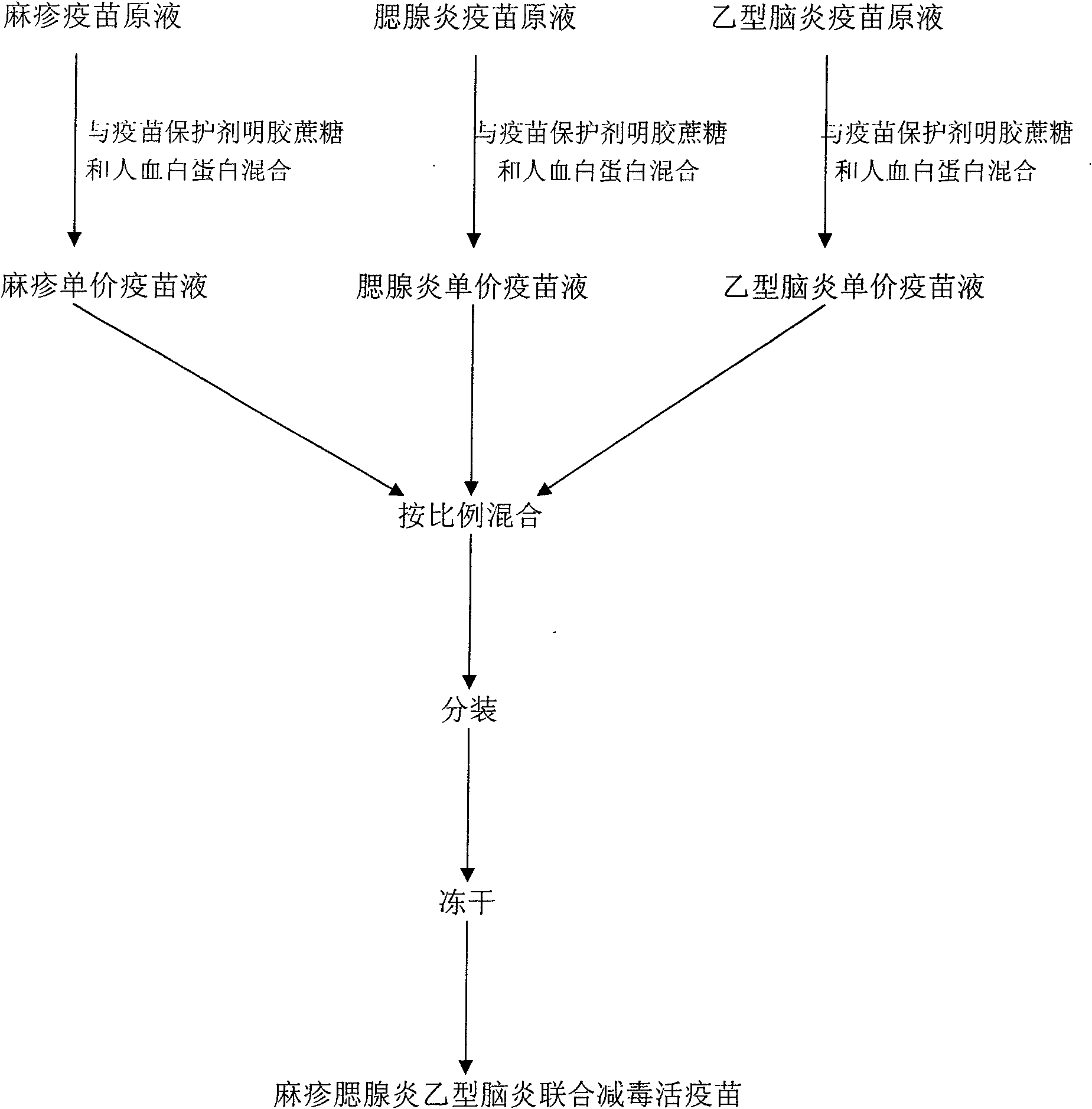
[0055] The preparation method of the measles mumps Japanese encephalitis joint attenuated live vaccine provided by the invention is as follows:
[0056] 1. Preparation of three kinds of monovalent vaccine stock solutions: in the vaccine production workshop of Wuhan Institute of Biological Products, which has passed the national GMP certification, according to the requirements of the three parts of the 2005 edition of the "Pharmacopoeia of the People's Republic of China", respectively
[0057] 1.1 According to the 2005 edition of the "Pharmacopoeia of the People's Republic of China" Part III, P98-99, the primary chicken embryo cells were inoculated with the measles Shanghai 191 vaccine strain, and the virus titer was prepared at 5.0 lgCCID 50 / ml~6.0lgCCID 50 / ml of measles vaccine stock solution;
[0058] 1.2 According to the requirements of the 2005 edition of "The Pharmacopoeia of the People's Republic of China", three volumes, P107-108, use mumps Wm 84 The vaccine strain ...
Embodiment 2
[0082] Preparation of combined vaccine of the present invention
[0083] It is operated in a vaccine production workshop certified by the national GMP.
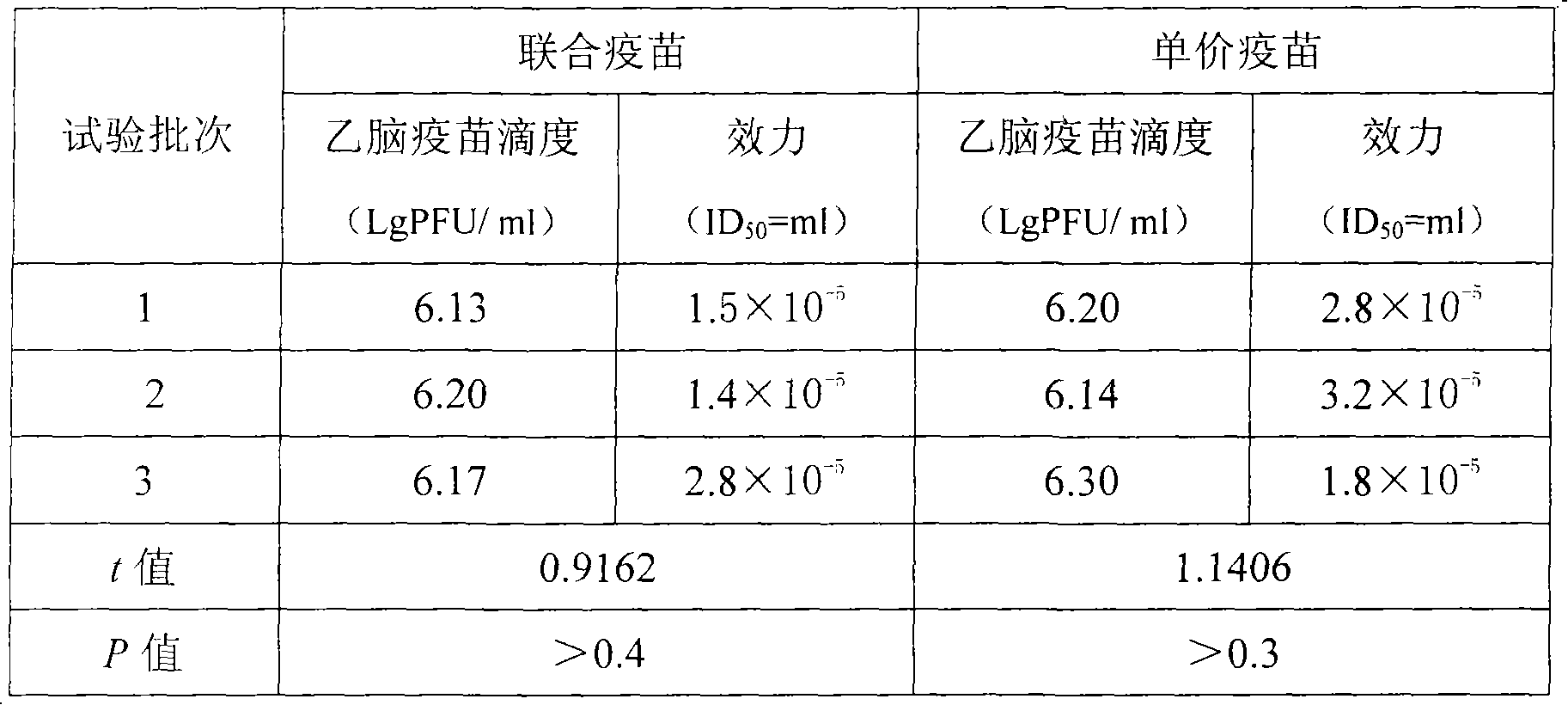
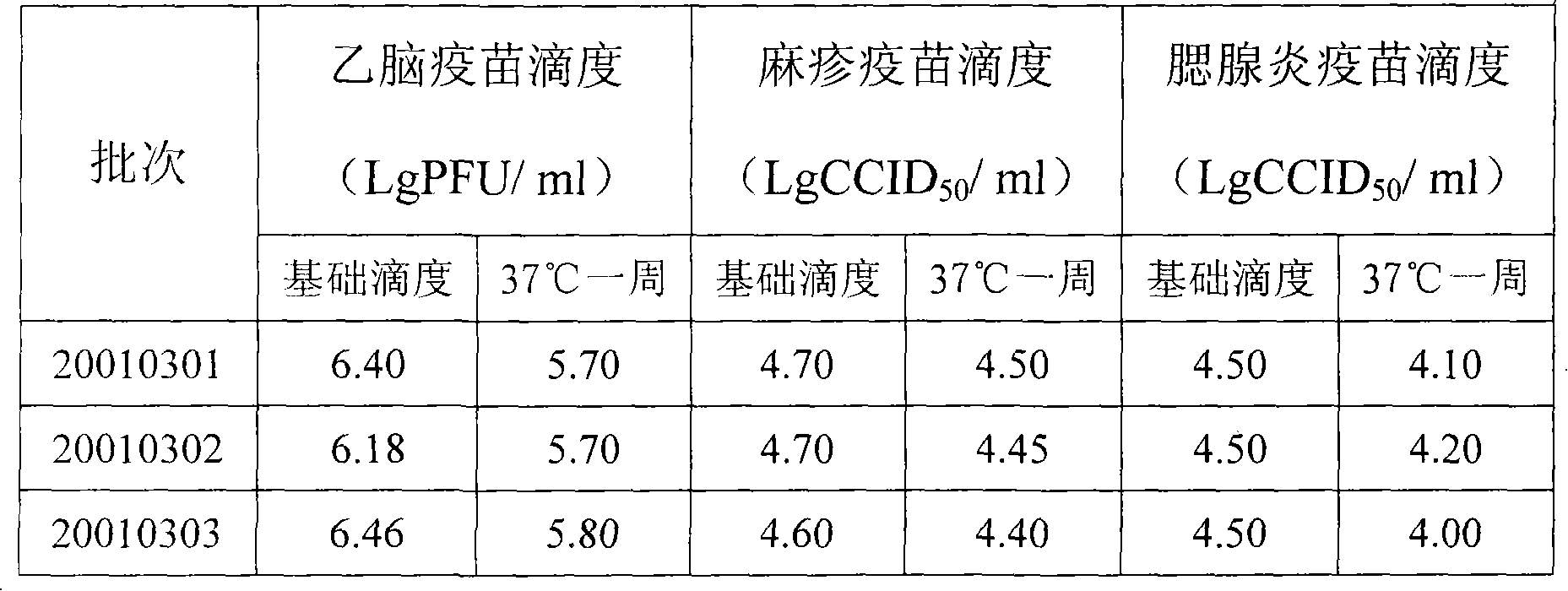
[0084] First prepare virus titer at 5.0lgCCID by the step 1 of embodiment 1 50 / ml of measles vaccine stock solution, the prepared virus titer is at 6.0lgCCID 50 The mumps vaccine stoste of / ml prepares the Japanese encephalitis vaccine stoste of virus titer at 7.0lgPFU / ml; Then prepare the vaccine protective agent according to the step 2 of embodiment 1; then press the 8% gelatin prepared in the step 2 of embodiment 1 Add 8600ml of the prepared measles vaccine stock solution to 1000ml of 40% sucrose protective agent, mix well, then add 400ml 20% human serum albumin, mix well. At this point, a monovalent measles vaccine solution with a volume of 10,000 ml has been prepared, containing a final concentration of 0.8% gelatin, 4% sucrose, and 0.8% human albumin. Prepare the monovalent mumps vaccine liquid of volume 10000ml and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com