Double-nuclear ring metal platinum complex near-infrared light-emitting material and application thereof
A technology of platinum complexes and cyclometals, applied in near-infrared polymer electroluminescent devices, in the field of near-infrared luminescent materials of binuclear cyclometal platinum complexes, can solve the problem of easy-to-quench luminescence and near-infrared electroluminescent materials Single variety, narrow selection range, etc., to achieve the effect of improving luminescence performance, enhancing carrier transport and capture capabilities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Synthesis of Arylformylhydrazine
[0077]
[0078] 1.1 Benzohydrazide
[0079] Add 40mL of ethanol and 0.025mol of methyl benzoate into a 100mL three-necked flask, heat to 60°C, slowly add 6.3g (0.1mol) of 80% hydrazine hydrate dropwise under stirring, after the drop is complete, reflux for 16h, and track the reaction by TLC. After the reaction was completed, most of the solvent was distilled off under reduced pressure, the reaction mixture was poured into ice water, and a large amount of white solid was precipitated, filtered with suction, washed with water, washed with a small amount of petroleum ether, dried in vacuum, and recrystallized from absolute ethanol to obtain 2.93 g of white flakes Crystal, yield 86.1%, m.p.113-115°C. 1H NMR (400MHz, CDCl 3 , TMS, δppm): 7.87(t, J=7.22, 1.39Hz, 2H); 7.49(s, 1H); 7.65(t, J=7.39, 7.32Hz, 1H); 7.56(t, J=7.78, 7.24 Hz, 2H).
[0080] 1.2 4-Hexyloxybenzohydrazide
[0081] The method is the same as 1.1, and 4.92 g of white...
Embodiment 2
[0093] Synthesis of 5-aryl-2-mercapto-1,3,4-oxadiazole
[0094]
[0095] 2.1 5-Phenyl-2-mercapto-1,3,4-oxadiazole (OXT)
[0096] In a 50mL flask, add 0.01mol benzohydrazide, 0.56g (0.01mol) KOH in 10mL of ethanol and 1.48g (0.02mol) of CS 2 . Stir and heat under reflux for 4 hours, follow the reaction by TLC. After the reaction, distill off the solvent under reduced pressure, add water to dissolve the solid, add 10% hydrochloric acid drop by drop to adjust the pH to 6-7, a large amount of solid precipitates, filter to obtain the crude product, and recrystallize from ethanol to obtain 1.45 g of white solid, yield: 81.2%. m.p.214~216°C. 1H NMR (400MHz, CDCl 3 , TMS, δppm): 7.93 (d, J=7.6Hz, 2H); 7.57 (d, J=6.84Hz, 1H); 7.51 (t, J=7.16, 7.76Hz, 2H); 13C NMR (400MHz, CDCl3 , TMS, δppm): 177.94, 162.67, 161.87, 128.41, 115.20, 114.39.
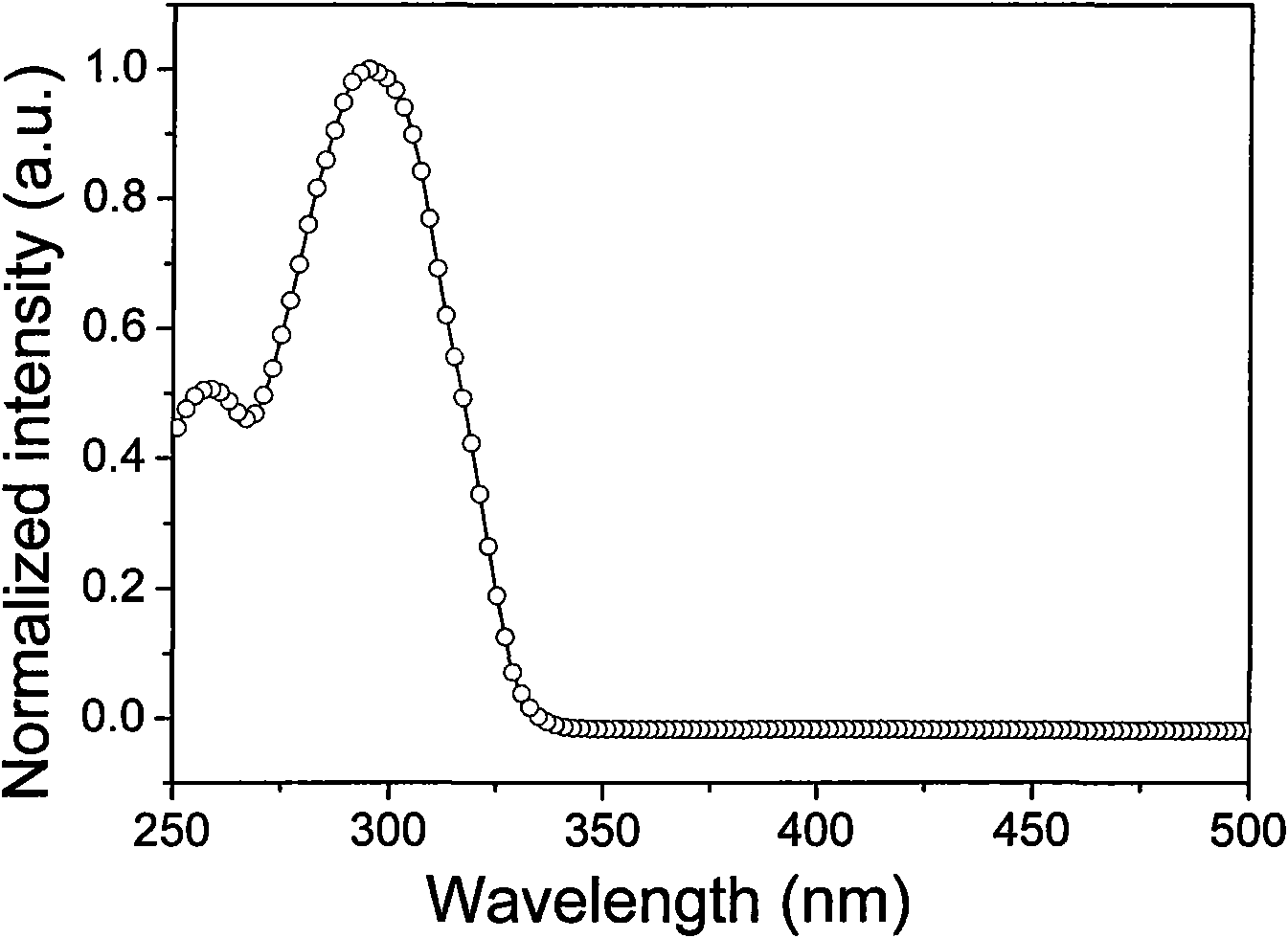
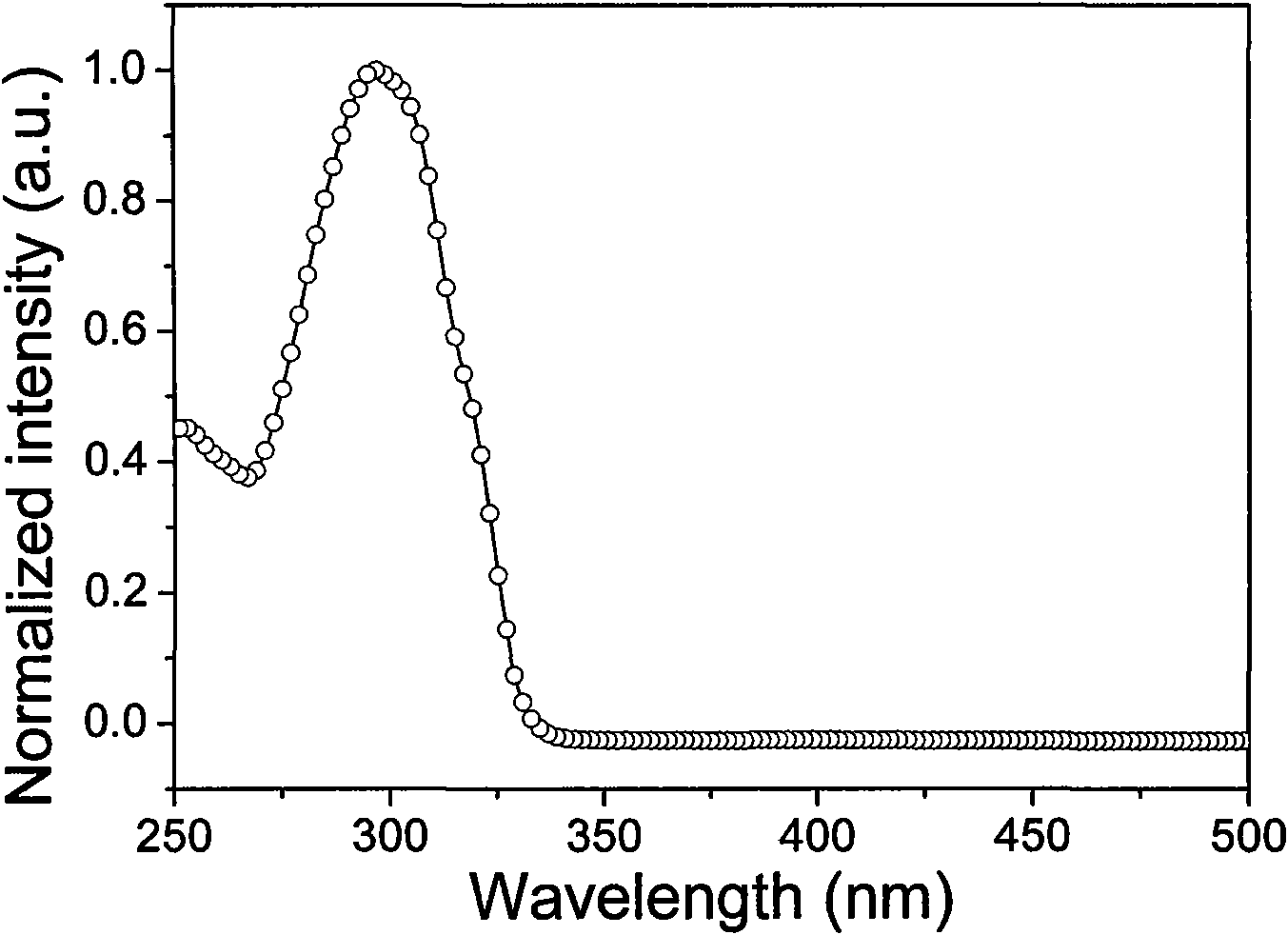
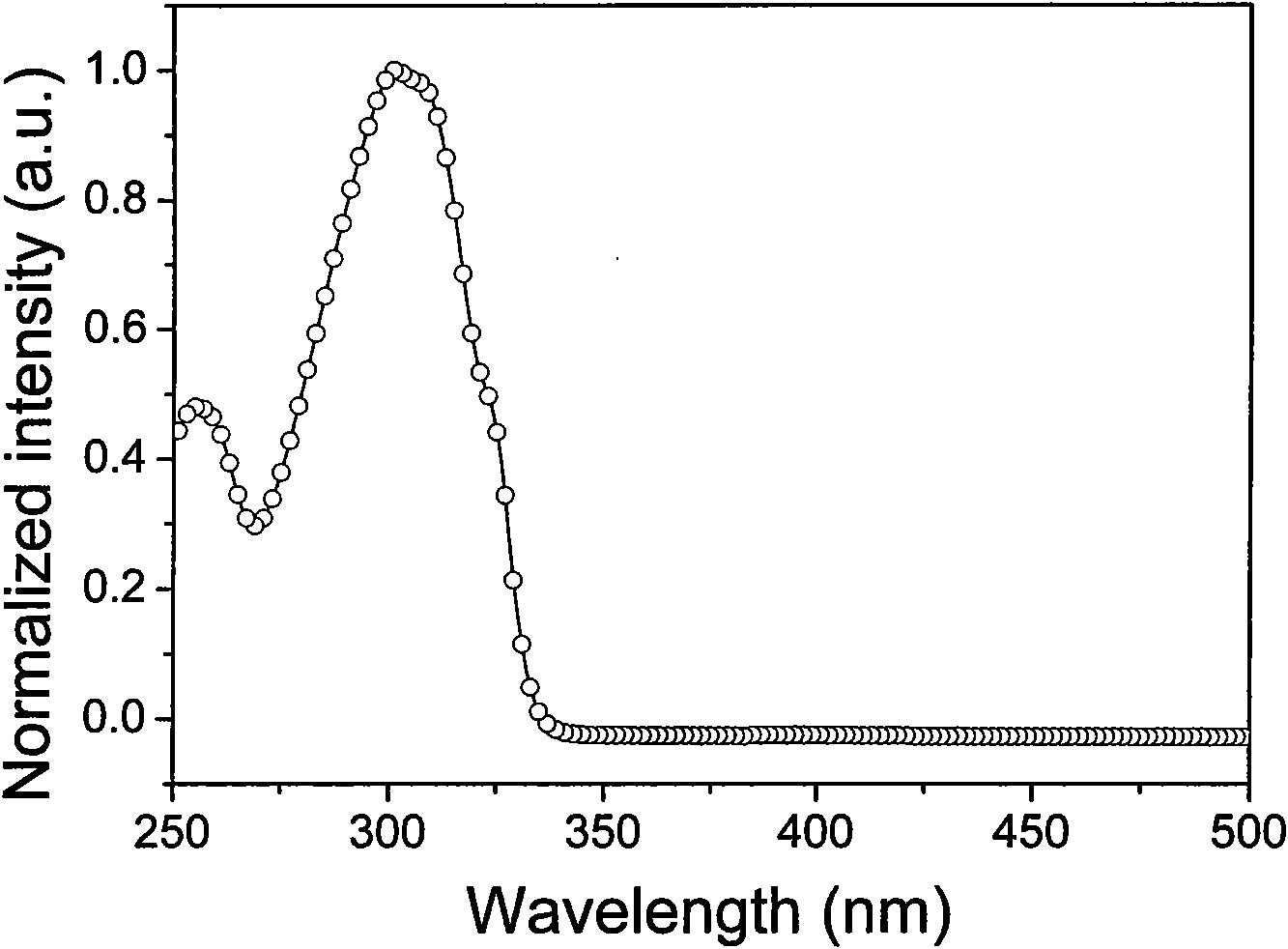
[0097] Ligand 5-phenyl-2-mercapto-1,3,4-oxadiazole (OXT) in dichloromethane solution (1×10 -5 The UV-Vis absorption spectrum peaks in M) ...
Embodiment 3
[0117] Synthesis of [(1-phenylisoquinoline)-C2′, N)]-[(1-phenylisoquinoline)-N]-chloroplatinum(piq)Pt(Hpiq)Cl
[0118]
[0119] Add 0.84g (2.0mmol) potassium chloroplatinite, 1.03g (5.0mmol) 1-phenylisoquinoline, 27mL ethylene glycol monomethyl ether, 9mL water in sequence in a 50mL three-necked flask, under nitrogen protection, keep the temperature at 80 React at ℃ for 16 hours, cool to room temperature, remove part of the solvent by distillation under reduced pressure, add a small amount of distilled water, a brown precipitate is formed, filter with suction, wash the obtained solid with water and n-hexane in turn, and dry in vacuo to obtain 1.0 g of a yellow solid with a yield of 77.6%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| emission peak | aaaaa | aaaaa |
| luminance | aaaaa | aaaaa |
| emission peak | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com