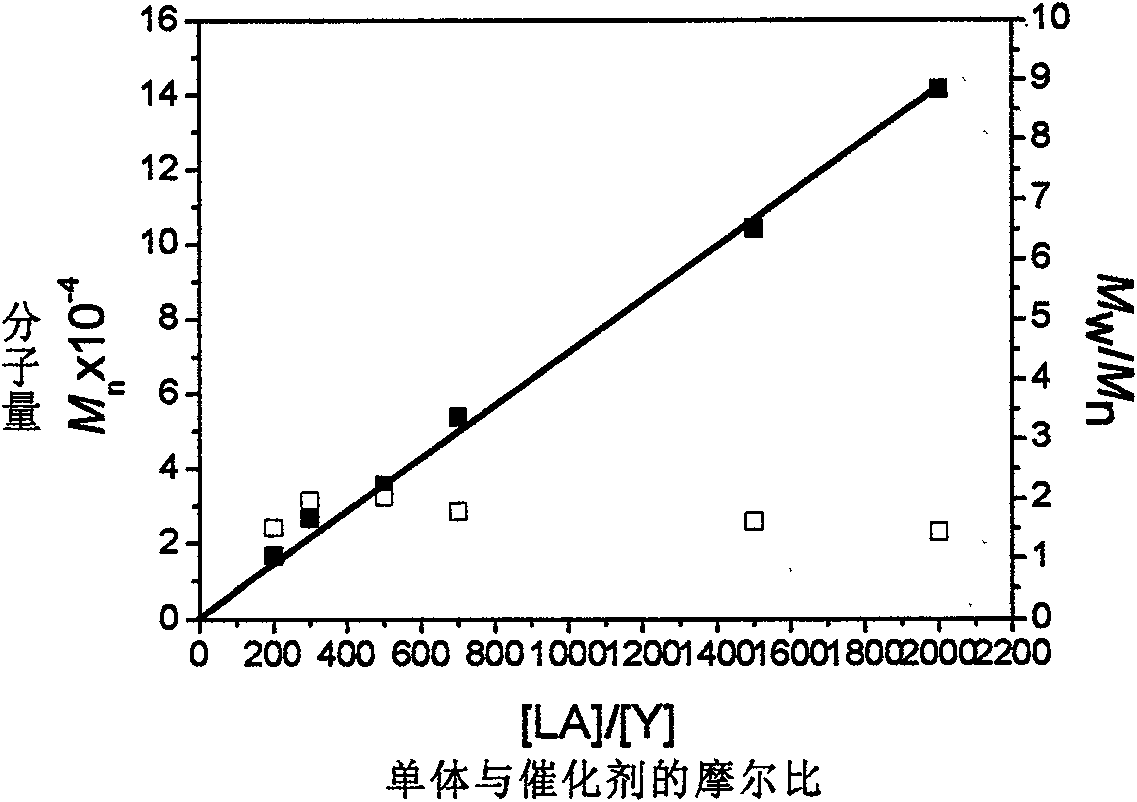
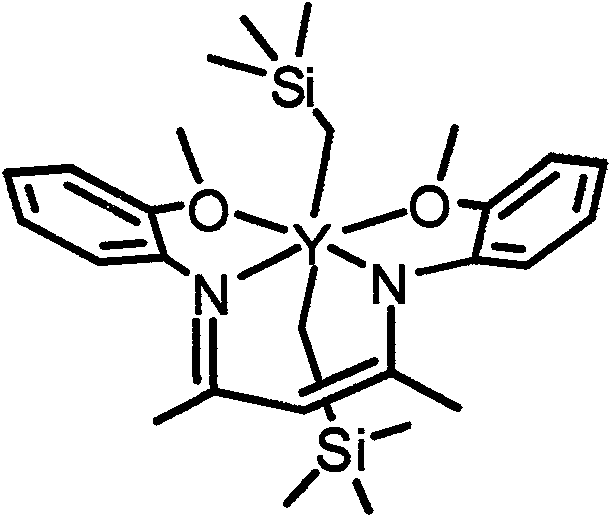
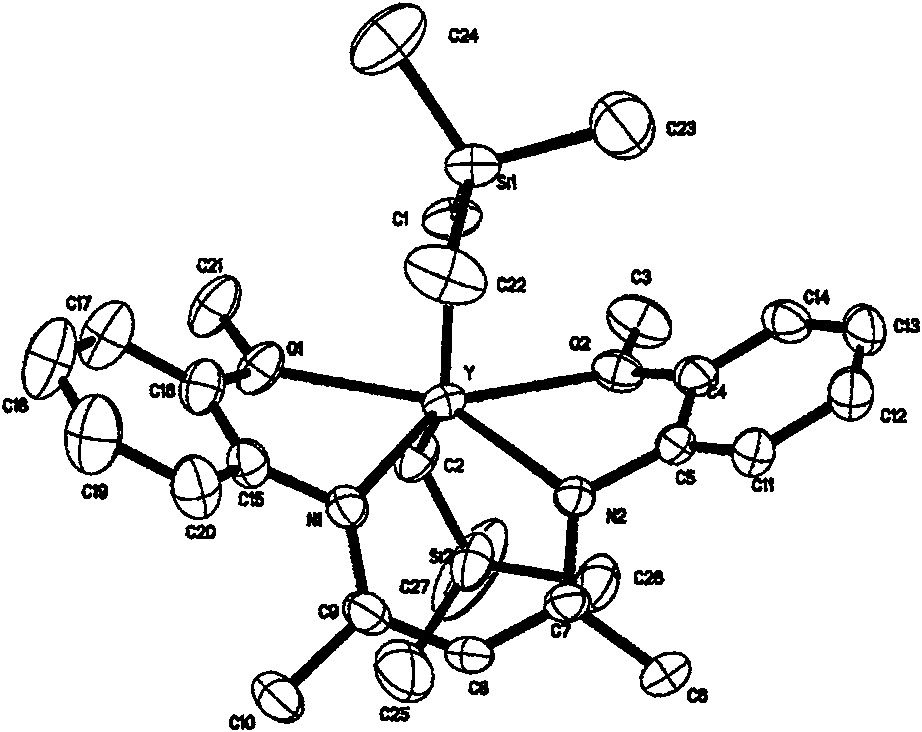
Rare earth alkyl coordination compound for configuration maintenance of catalytic levorotatory lactide and ring-opening polymerization as well as preparation method and use method thereof
A technology of L-lactide and alkyl complexes, applied in the field of rare earth alkyl complexes, can solve the problems of non-renewable petroleum resources, resource shortage, white pollution and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0083] Preparation Example 1 Preparation of Complex 12
[0084] (1) Preparation of ligand 4 used in complex 12, the reaction process is as follows:
[0085]
[0086] Ligand 4
[0087] Dissolve 2,4-pentanedione (5mL, 0.048mol), 2-methoxyaniline (12mL, 0.106mol) in 30mL toluene, add a catalytic amount of p-toluenesulfonic acid, and use a The reflux device of the water separator is heated and refluxed at 120 degrees for 4 hours. The remaining toluene was distilled off under reduced pressure, and the resulting brown oily solid was cooled to room temperature. The oily solid was crushed in a small amount of methanol solvent, then suction filtered and washed 5 times with cold methanol. Recrystallized three times from a mixed solution of methanol / n-hexane (4 / 1), and finally obtained a light yellow solid, ie ligand 4 (2.60 g), with a yield of 87.3%. With deuterated chloroform (CDCl 3 ) is a 300 MHz nuclear magnetic resonance instrument (hydrogen spectrum, 1 H NMR) characterized...
preparation Embodiment 2
[0092] Preparation Example 2 Preparation of Complex 15
[0093] (1) Preparation of ligand 5 used in complex 15, the reaction process is as follows:
[0094]
[0095] Ligand 5
[0096] Dissolve 2,4-pentanedione (5mL, 0.048mol), 2,6-diisopropylaniline (9.65g, 0.106mol) in 30mL of toluene, add a catalytic amount of p-toluenesulfonic acid, and Next, use a reflux device with a water separator to heat and reflux at 120 degrees for 4 hours. The remaining toluene was distilled off under reduced pressure, and the resulting brown oily solid was cooled to room temperature. The oily solid was crushed in a small amount of methanol solvent, then suction filtered and washed 5 times with cold methanol. Recrystallized three times from a mixed solution of methanol / n-hexane (4 / 1) to finally obtain a light yellow solid, Ligand 5 (12.00 g), with a yield of 90.0%.
[0097] (2) The preparation of complex 15, its reaction process is shown in the following formula
[0098]
[0099] Ligand 5 ...
preparation Embodiment 3
[0101] Preparation Example 3 Preparation of Complex 20
[0102] (1) The ligand used in complex 20 is ligand 4.
[0103] (2) The preparation of complex 20, its reaction process is shown in the following formula:
[0104]
[0105] Ligand 4 Complex 20
[0106] In a glove box, dissolve ligand 4 (0.26 g, 0.84 mmol) in 5 mL of n-hexane, cool to -30 °C, add equimolar Sc(CH 2 SiMe 3 ) 3 (THF) 2 (0.377g, 0.84mmol) in 5mL cold n-hexane solution (-30°C); control the reaction temperature at -30°C, after 0.5h of reaction, remove 2 / 3 of the solvent under reduced pressure, and freeze to obtain light yellow crystals, namely the complex 20 (0.36 g), the yield was 80%. deuterated benzene (C 6 D. 6 ) is the proton spectrum of the reagent with nuclear magnetic resonance (( 1 HNMR), 400 MHz) characterized the structure of the ligand. 1 H NMR (400MHZ, C 6 D. 6 ): δ=6.87-6.80 (td, J=1.7Hz, 1.4Hz, 1.6Hz, 2H, 2-OMe-C 6 h 4 -βH), 6.80-6.71 (td, J=1.2Hz, 1.1Hz, 1.2Hz, 2H, 2-OMe-C 6 h ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com