Medicinal composition containing felodipine and metoprolol
A kind of technology of felodipine and composition, applied in the field of solid pharmaceutical composition containing felodipine and metoprolol
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
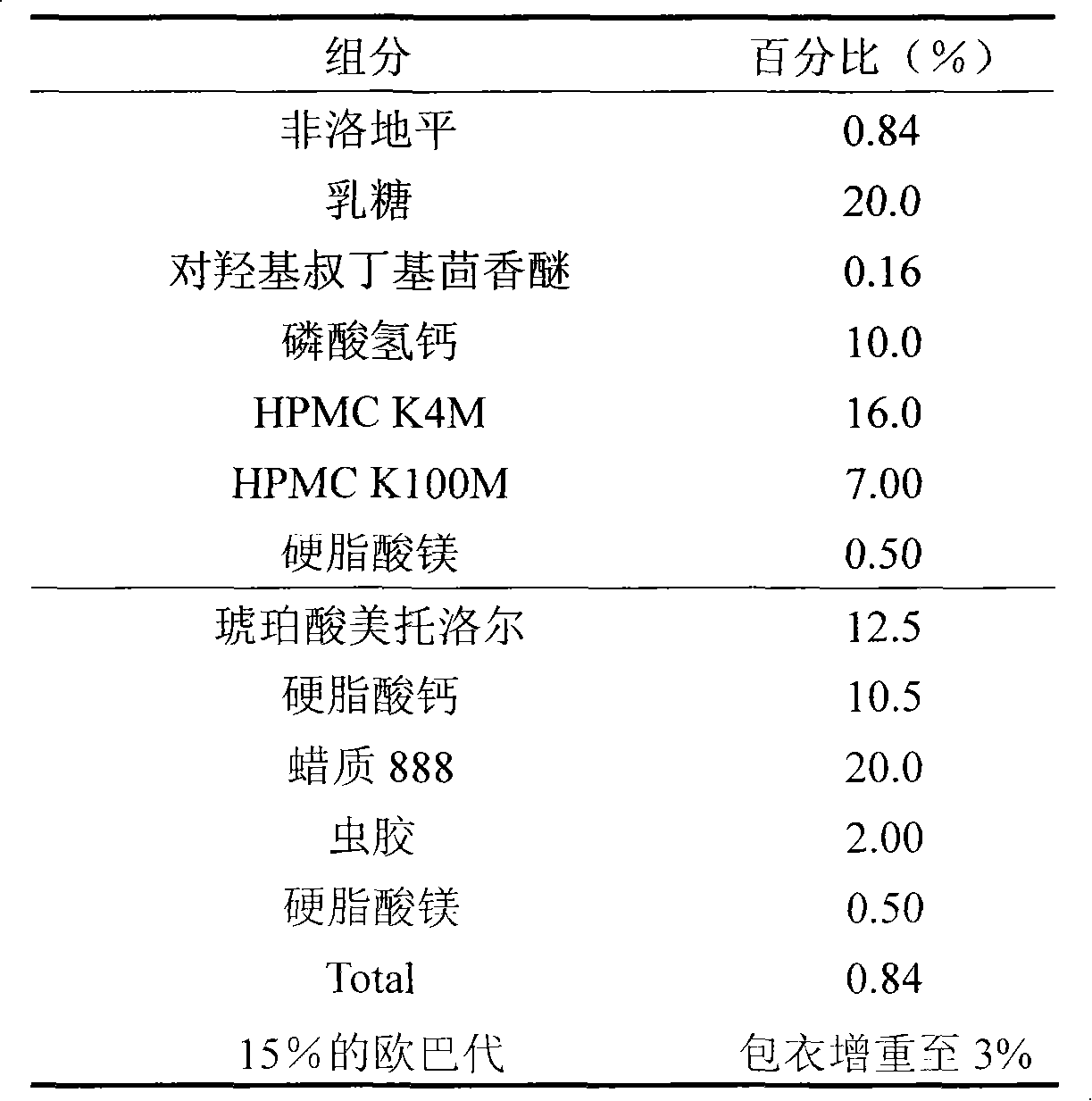
[0023]
[0024] Preparation:
[0025] Weigh the prescribed amount of felodipine and p-hydroxytert-butylanisole, add a certain amount of ethanol to dissolve, add lactose to mix, then mix with calcium hydrogen phosphate and hypromellose, add 50% alcohol water to make granules , dry, add magnesium stearate and mix well, and set aside; another prescription amount of metoprolol succinate and calcium stearate is crushed together, passed through a 200-mesh sieve, mixed with wax 888, and ethanol with shellac The solution is granulated, dried, added with magnesium stearate and mixed evenly, together with the above-mentioned felodipine granules, it is compressed into tablets by a double-layer tablet press. Coating with 15% Opadry solution, that is.
Embodiment 2
[0027]
[0028] Preparation:
[0029] Weigh the prescribed amount of felodipine and p-hydroxytert-butylanisole, add a certain amount of ethanol to dissolve, add lactose to mix, then mix with calcium hydrogen phosphate and hypromellose, add 50% alcohol water to make granules , dry, add magnesium stearate and mix well, and set aside; another prescription amount of metoprolol succinate and magnesium stearate is weighed and pulverized together, passed through a 80-mesh sieve, mixed with wax 888, and ethanol with shellac The solution is granulated, dried, added with magnesium stearate and mixed evenly, together with the above-mentioned felodipine granules, it is compressed into tablets by a double-layer tablet press. Coating with 15% Opadry solution, that is.
Embodiment 3
[0031]
[0032] Preparation:
[0033] Weigh the prescribed amount of felodipine and p-hydroxytert-butylanisole, add a certain amount of ethanol to dissolve, add lactose to mix, then mix with calcium hydrogen phosphate and hypromellose, add 50% alcohol water to make granules , dried, added magnesium stearate and mixed, and set aside; another prescription amount of metoprolol succinate was crushed with stearyl alcohol, passed through a 65-mesh sieve, mixed with waxy 888, ethyl cellulose, added insecticide The ethanol solution of the gum is granulated, dried, mixed with magnesium stearate, and compressed with the above-mentioned felodipine granules using a double-layer tablet press. Coating with 15% Opadry solution, that is.
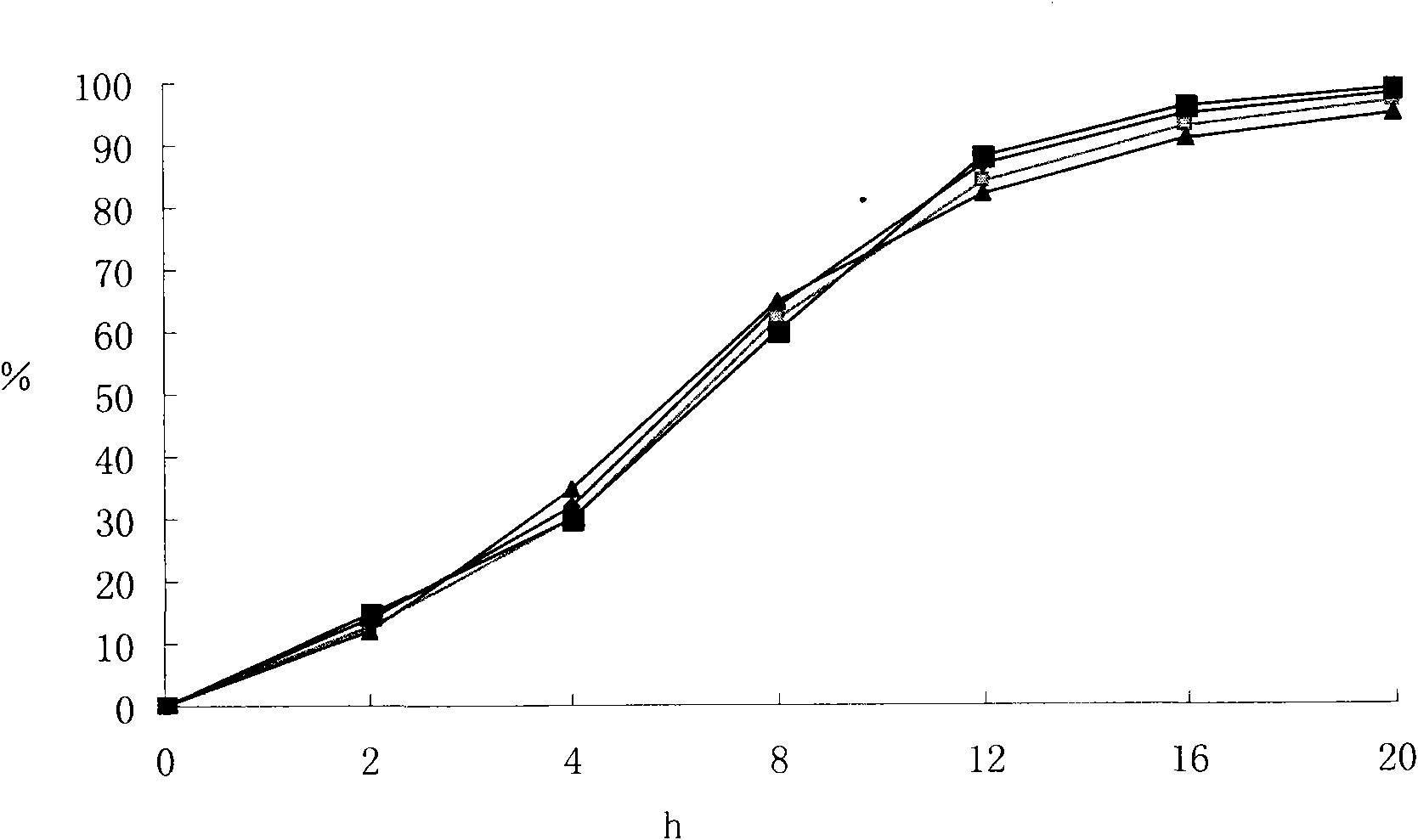
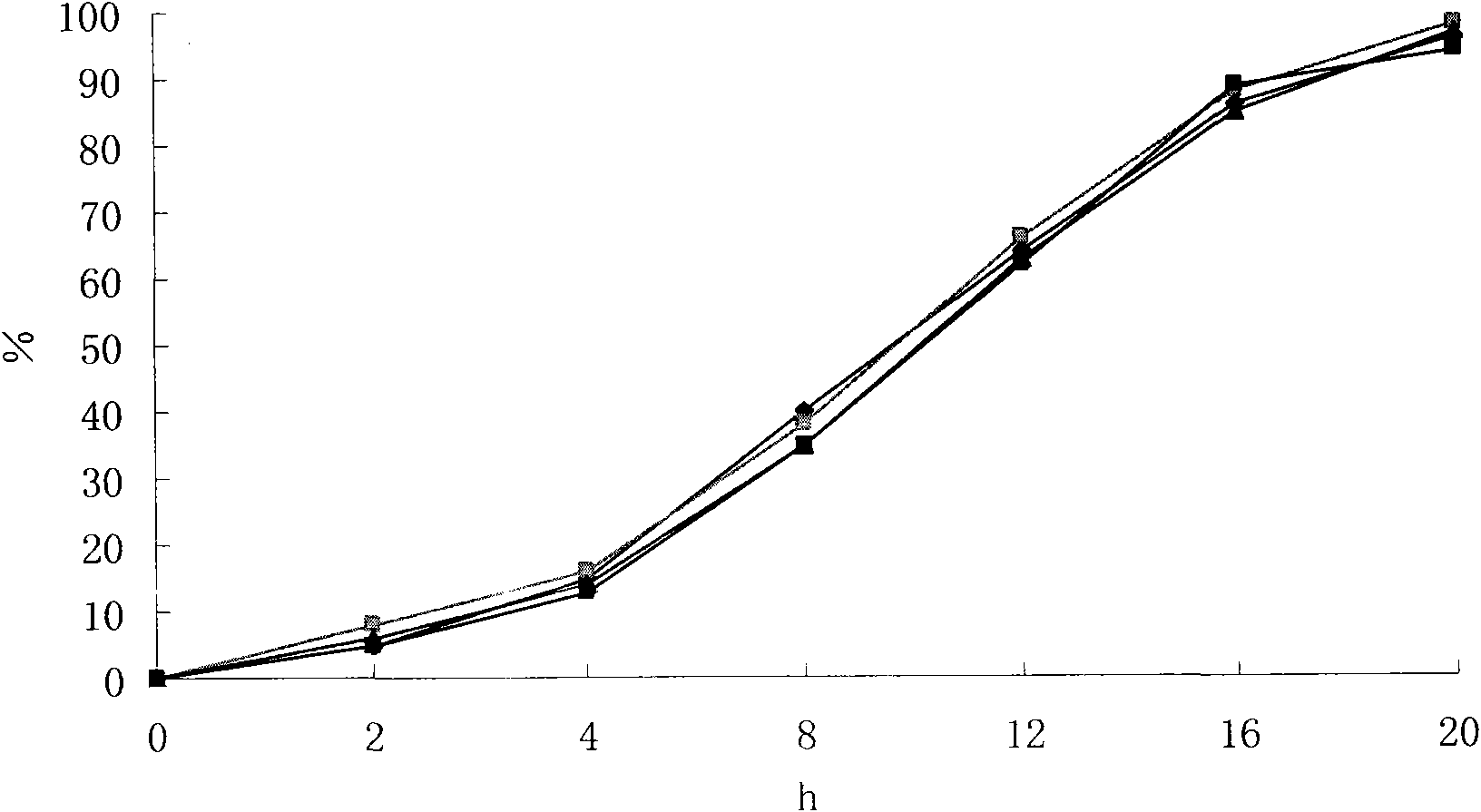
[0034] The above-mentioned embodiment 1,2,3 and the compound felodipine metoprolol slow-release tablet of commercially available trade name LOGIMAX are in the phosphate buffer saline buffer of 6.5 respectively in the pH of 1% SDS, according to Chinese P...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com