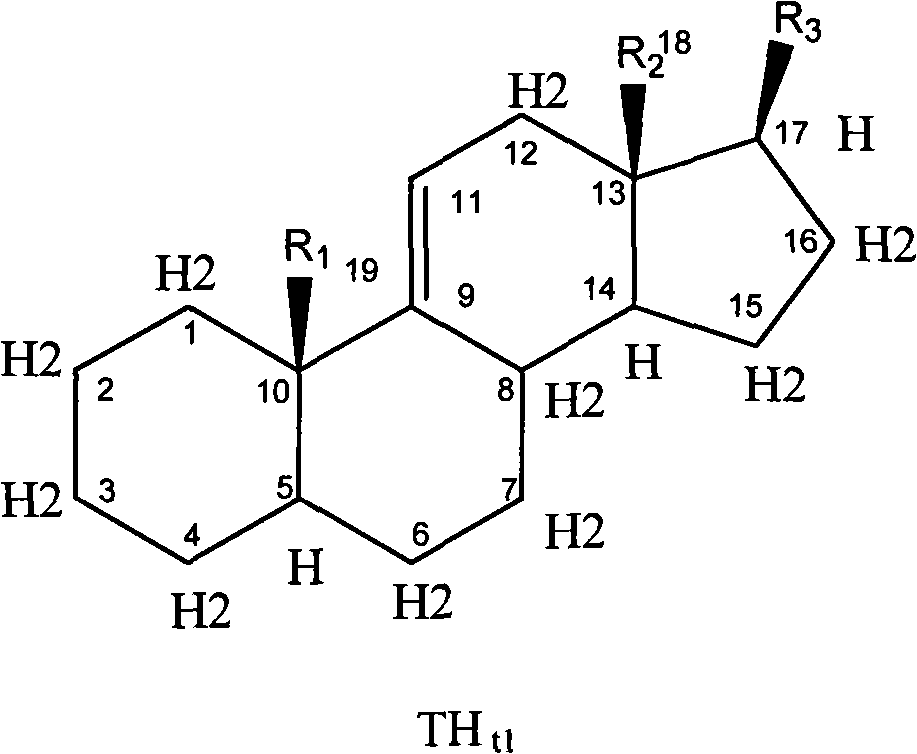
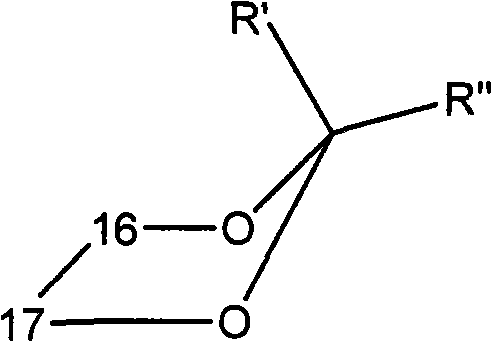
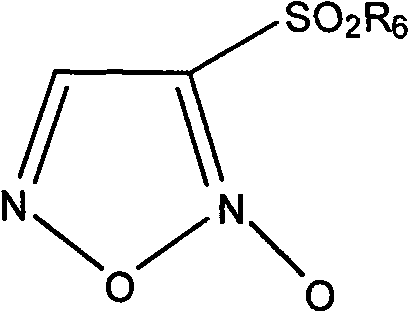
Nitrate medicament for inhibiting angiogenesis
A technology of drugs and compounds, applied in the field of medicine, can solve problems such as ineffective regression of new blood vessels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0175] Example 1 4-(pregna-4,9(11)-diene-3,20-diketone-21-hydroxy-21-acetate-17-oxo)-4-oxo-butyric acid-2- [2-(3-Benzenesulfonyl-1,2,5-oxadiazole-2-oxide-4-oxy)ethoxy]ethyl ester
[0176]
[0177] 1) 4-(pregna-4,9(11)-diene-3,20-diketone-21-hydroxy-21-acetate-17-oxo)-4-oxo-butanoic acid (3.1) synthesis
[0178] Dissolve 15mmol pregna-4,9(11)-diene-3,20-diketone-17,21-dihydroxy-21-acetate in 80ml pyridine, add 0.30mmol DMAP, add 30mmol succinic anhydride, heat to reflux After 5 hours, the reaction was stopped. After cooling, it was poured into 200 ml of saturated ice-cold brine, and the pH was adjusted to 5 with hydrochloric acid. A white solid precipitated out after resting, and was filtered and washed with water to obtain 8.1 mmol of the product.
[0179] 2) 4-(pregna-4,9(11)-diene-3,20-diketone-21-hydroxyl-21-acetate-17-oxygen)-4-oxo-butyric acid-2-[ Synthesis of 2-(3-Benzenesulfonyl-1,2,5-oxadiazole-2-oxide-4-oxo-)ethoxy]ethyl ester (9.1)
[0180] Dissolve 8mmol of t...
Embodiment 2
[0185] Example 2 4-(pregna-4,9(11)-diene-3,20-diketone-21-hydroxyl-17-oxo)-4-oxo-butanoic acid-2-[2-(3 -Benzenesulfonyl-1,2,5-oxadiazole-2-oxide-4-oxo)ethoxy]ethyl ester
[0186]
[0187] 4mmol 4-(pregna-4,9(11)-diene-3,20-diketone-21-hydroxy-21-acetate-17-oxo)-4-oxo-butyric acid-2-[ 2-(3-Benzenesulfonyl-1,2,5-oxadiazole-2-oxide-4-oxo-)ethoxy]ethyl ester was dissolved in 10ml of methanol and chloroform (1:1), under nitrogen protection Add dropwise an aqueous solution of sodium carbonate (0.0045mol) saturated at 0°C at 0°C, stir for 10 hours, adjust the pH of the reaction system to neutral with hydrochloric acid, concentrate under pressure, remove chloroform, and dilute the solution in 40ml of ice water , filtered, and dried to obtain the crude product of the title compound. The crude product was subjected to column chromatography, eluting with methanol and chloroform (1:4) as the mobile phase, and the main compound was concentrated under reduced pressure, washed into meth...
Embodiment 3
[0192] Example 3 2-(pregna-4,9(11)-diene-3,20-diketone-17-hydroxyl-21-oxo-)-2-oxo-acetic acid-2-[2-(3 -Benzenesulfonyl-1,2,5-oxadiazole-2-oxide-4-oxo-)ethoxy]ethyl ester
[0193]
[0194] 1) Synthesis of 2-(pregna-4,9(11)-diene-3,20-diketone-17-hydroxyl-21-oxo-)-2-oxo-acetic acid (3.3)
[0195] Dissolve 15mmol of pregna-4,9(11)-diene-3,20-diketone-17,21-dihydroxyl in 80ml of pyridine, add 30mmol of oxalic anhydride, heat and reflux for 5 hours to stop the reaction, after cooling Pour it into 200 ml of saturated ice-cold brine, adjust the pH to 5 with hydrochloric acid, a white solid precipitates out after resting, filter and wash with water to obtain 10.3 mmol of the product.
[0196] 2) 2-(pregna-4,9(11)-diene-3,20-diketone-17-hydroxyl-21-oxo-)-2-oxo-acetic acid-2-[2-(3- Synthesis of Benzenesulfonyl-1,2,5-oxadiazole-2-oxide-4-oxo-)ethoxy]ethyl ester (9.3)
[0197] Dissolve 10mmol of the compound obtained in step 1) in 300ml of anhydrous dichloromethane, add 11mmol of co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com