Phenanthrenequinone compound, electrode active material, and electrical storage device
一种电极活性物质、化合物的技术,应用在活性物质电极、混合电容器、混合电容器电极等方向,能够解决难电极活性物质、使用等问题,达到高输出功率、循环特性优异、高容量的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
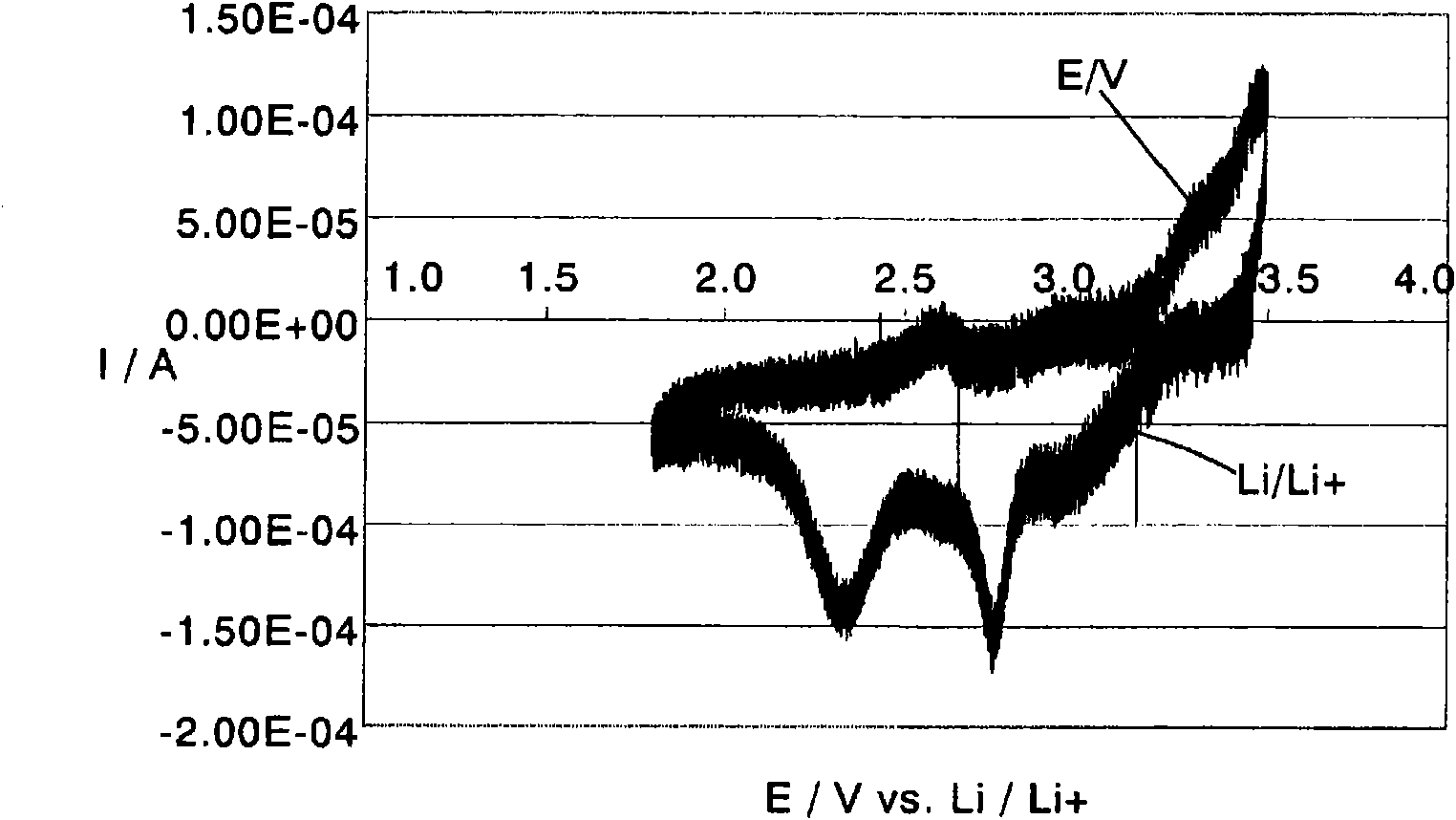
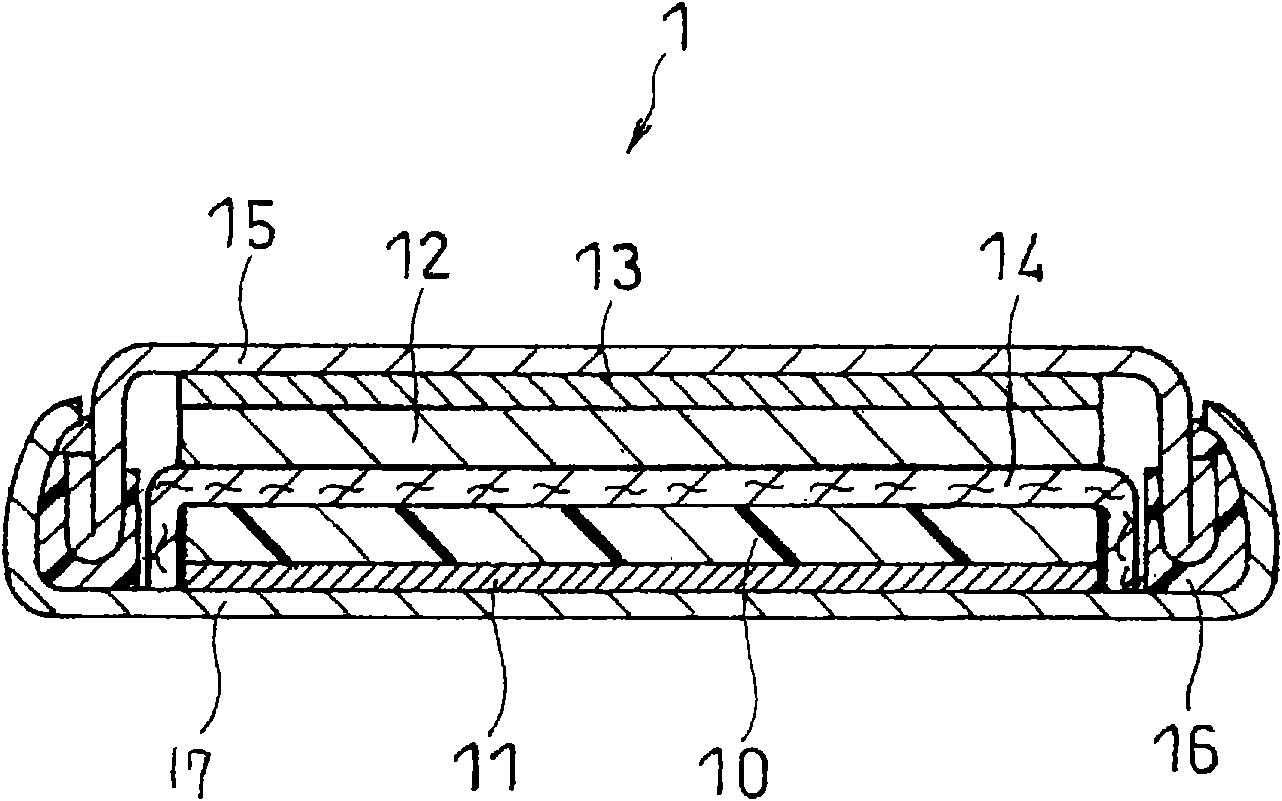
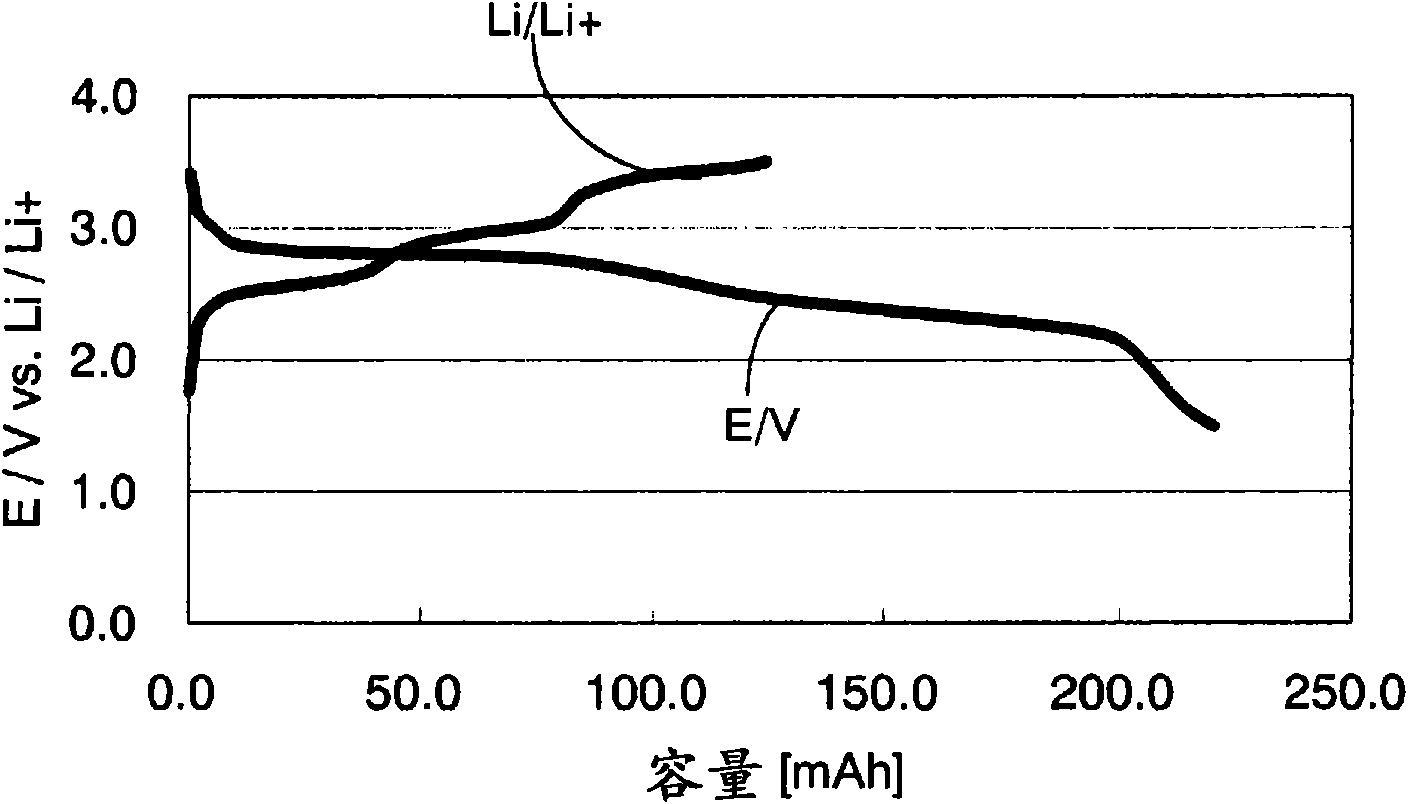
Image
Examples
Embodiment 1
[0139] (1) Synthesis of 2-iodophenanthrene-9,10-dione
[0140] In an eggplant-shaped flask with a capacity of 200ml, add 10.0g of 9,10-phenanthrenequinone, 54.8g of trifluoroacetic acid and 21.6g of N-iodosuccinimide at room temperature, raise the temperature to 35°C, and then stir in an argon atmosphere 36 hours. The reaction mixture was poured into ice water, and the precipitated solid was filtered off and recrystallized using tetrahydrofuran to obtain 12.1 g of 2-iodophenanthrene-9,10-dione as an orange solid (yield 75%).
[0141] (2) Synthesis of 9,10-bis(tert-butyldimethylsilyloxy)-2-iodo-9,10-dihydrophenanthrene
[0142] In an eggplant-shaped flask with a capacity of 200ml, add 1.0g of 2-iodophenanthrene-9,10-dione, 3.0g of zinc powder, 100ml of dichloromethane, N,N,N',N'-tetramethylethylenediamine 1.8 g and 1.9 g of tert-butyldimethylsilyl chloride (hereinafter referred to as "TBDMSCl") were stirred overnight at room temperature in an argon atmosphere. 1.0 g of TBDMS...
Embodiment 2
[0151] (1) 9,10-bis(tert-butyldimethylsilyloxy)-2-(4,4,5,5-tetramethyl-1,3,2-dioxaborolane Synthesis of -2-yl)-9,10-dihydrophenanthrenequinone
[0152] In a 100ml eggplant-shaped flask, add 9,10-bis(tert-butyldimethylsilyloxy)-2-iodo-9,10-dihydrophenanthrenequinone 6.4g and dehydrated ether 50ml, cool to -78°C. 9.5 ml of tert-butyllithium (1.31 mole n-pentane solution) was added dropwise to the cooled solution, and the mixture was stirred for 1 hour. 2.6 ml of 2-isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane was added to the mixture, and the temperature was further gradually raised to room temperature while stirring. The resulting reaction mixture was subjected to column purification (filler: neutral granular silica gel, solvent: hexane: ethyl acetate = 10:1), and concentrated to obtain the compound represented by the following chemical structural formula as a white solid: 9,10-bis(tert-butyldimethylsilyloxy)-2-(4,4,5,5-tetramethyl-1,3,2-dioxaborolane-2- Base)-9,10-dih...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| UV absorption wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com