Method for preparing CuCl and Cu loaded solid phase catalyst
A technology of solid catalyst and solid carrier, applied in molecular sieve catalysts, chemical instruments and methods, physical/chemical process catalysts, etc., to achieve the effects of avoiding waste water and waste gas, low cost, and reducing intermediate preparation processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
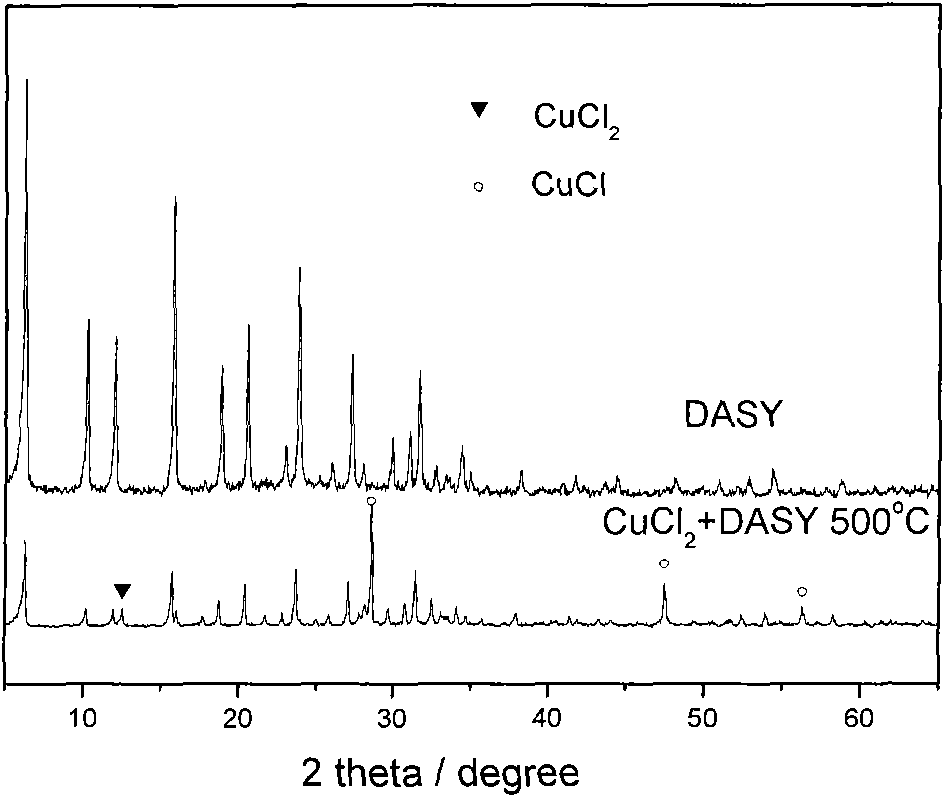
[0016] CuCl 2 After mixing and grinding with the hydrogen-type Y molecular sieve carrier at a mass ratio of 1:1, take 6g of the mixed sample and put it into a quartz tank, and put it into a N 2 In a protection furnace, heat up to 500°C at a heating rate of 2°C / min, then react at a constant temperature for 4 hours, then drop to room temperature, take out a sample and weigh 3.5g, which is the prepared loaded CuCl and Cu I solid catalyst.
[0017] The results of phase analysis by X-ray diffractometer are shown in figure 1 . It can be seen from Figure 1 that copper in the prepared catalyst mainly exists in the form of cuprous chloride and is dispersed on the surface of Y molecular sieve, while it has been reported in the literature that cuprous chloride can exchange ions with H-type Y molecular sieve at 300°C-500°C form Cu I / Y active center. Therefore, the prepared catalysts were Y molecular sieve loaded CuCl and loaded Cu I solid catalyst.
Embodiment 2
[0019] CuCl 2 After mixing and grinding with A molecular sieve carrier at a mass ratio of 1:2, take 6g of the mixed sample and put it into a quartz tank, put it into an Ar protection furnace, heat it to 400°C at a heating rate of 2°C / min, and then react at a constant temperature for 3 hour, then down to room temperature, take out and weigh 3.6g, which is the prepared A molecular sieve loaded CuCl and loaded Cu I solid catalyst.
Embodiment 3
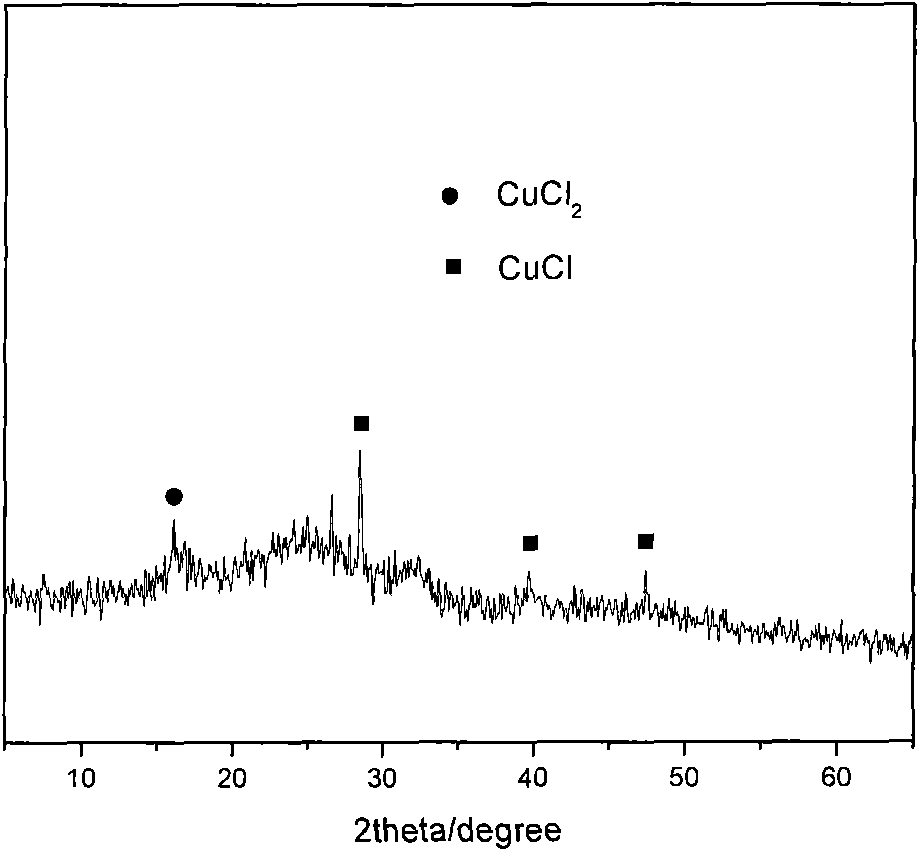
[0021] CuCl 2 with SiO 2 -Al 2 o 3 After the carrier was mixed and ground evenly at a mass ratio of 1:10, 6 g of the mixed sample was placed in a quartz tank, placed in a He protection furnace, heated to 600 °C at a heating rate of 5 °C / min, and then reacted at a constant temperature for 5 hours. Then drop to room temperature, take out and weigh 3.3g, which is the prepared SiO 2 -Al 2 o 3 Carrier loaded CuCl and loaded Cu I solid catalyst. Catalysts were analyzed by X-ray diffractometer figure 2 :
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com