Method for synthesizing stilbenoids by hydrochloric acid heterogeneous chlorination
A stilbene compound and heterogeneous technology, which is applied in the field of chlorination of hydrochloric acid for synthesizing stilbene compounds in heterogeneous phase, can solve the problems of separation difficulty, environmental damage, pollution and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 21-25
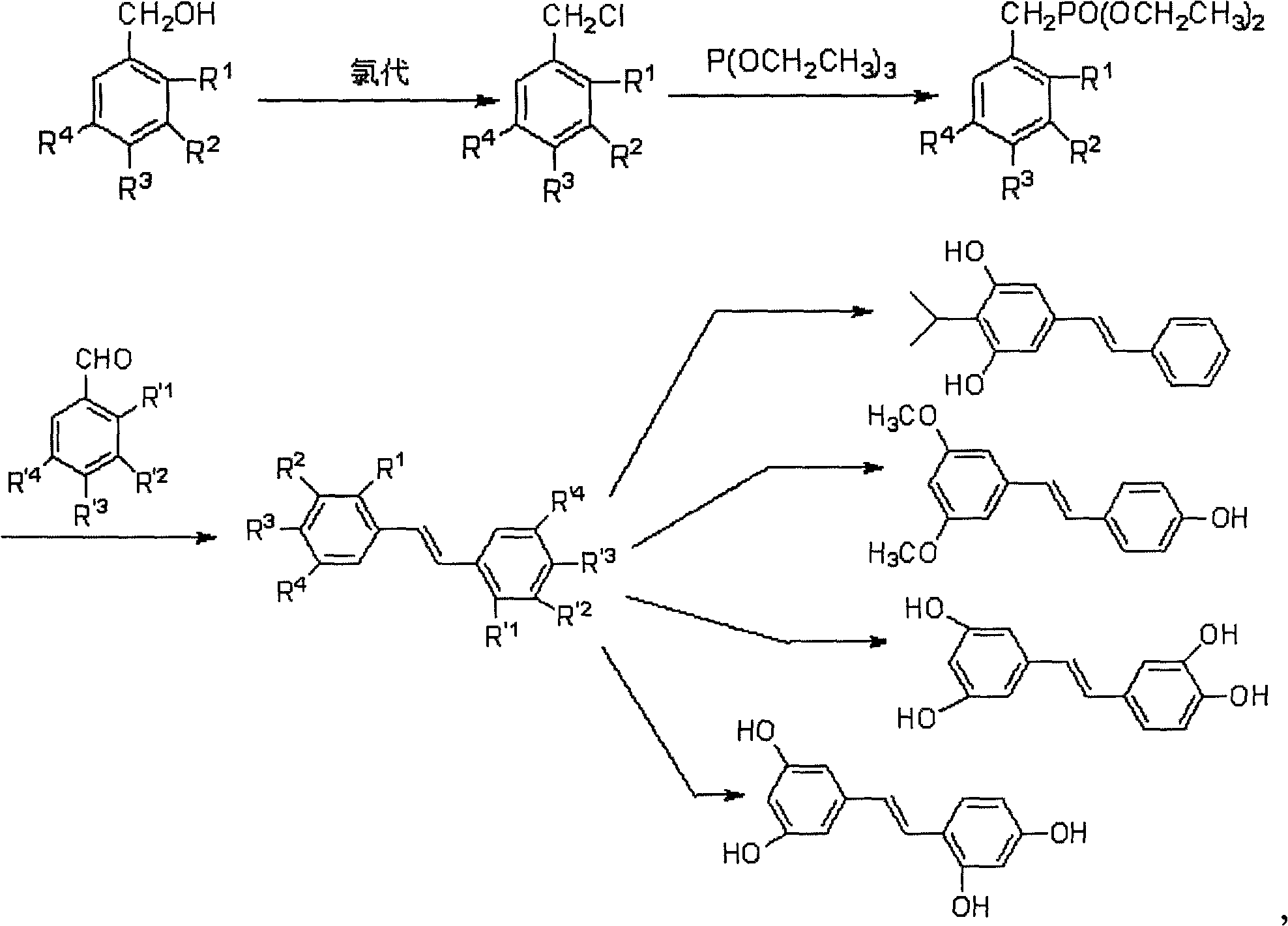
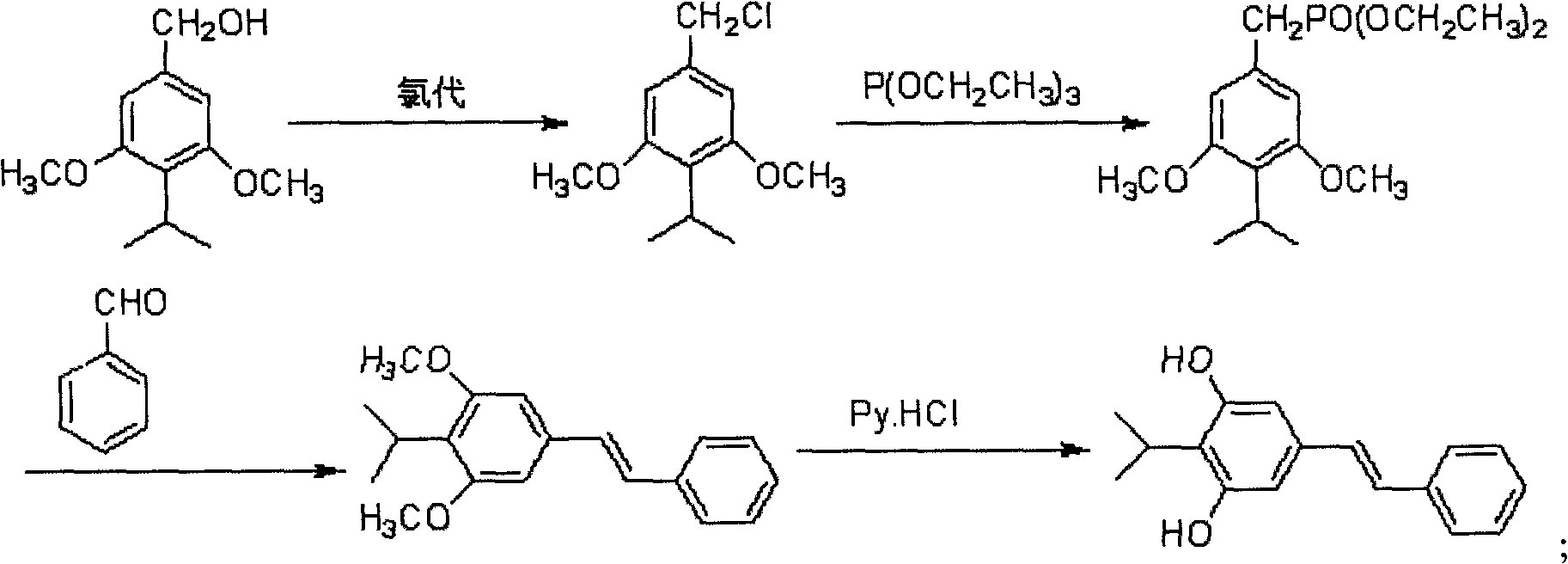
[0096] (2) When R 1 , R 3 , R' 1 , R' 2 , R' 4 is hydrogen; R 2 , R 4 is methoxy; R' 3 When it is an acetoxy group or an alkyl siloxy group, the final product is the structure of pterostylbene; the method is a method for synthesizing pterostilbene by hydrochloric acid heterogeneous chlorination, as in Examples 21-25;
[0097] (3) When R 1 , R 3 , R' 1 , R' 2 is hydrogen; R2 , R 4 , R' 3 , R' 4 When it is a methoxyl group, the final product is picetanol; the method is the first method for synthesizing picatanol by hydrochloric acid heterogeneous chlorination, as in Examples 31-35;
[0098] (4) When R 1 , R 2 , R' 1 , R' 3 is hydrogen; R 3 , R 4 , R' 2 , R' 4 When it is a methoxyl group, the final product is picetanol; the method is the second method for synthesizing picetanol with hydrochloric acid heterogeneous chlorination, as in Examples 41-45;
[0099] (5) When R 1 , R 3 , R' 2 , R' 4 is hydrogen; R 2 , R 4 , R' 1 , R' 3 When it is a methoxyl g...
Embodiment 41-45
[0143] Example 41-45 The second method of heterogeneous chlorination of hydrochloric acid to synthesize piceatanol
[0144]
[0145]
[0146] The method two of above-mentioned heterogeneous chlorination of hydrochloric acid to synthesize picatanol, the reaction scheme is as formula (V):
[0147]
[0148] Formula (V)
[0149] The method two of the above-mentioned heterogeneous chlorination of hydrochloric acid to synthesize piceatanol is carried out according to the following steps:
[0150] (41) Preparation A4: 3,4-dimethoxybenzyl chloride
[0151] Take 3,4-dimethoxybenzyl alcohol, add solvent, and slowly drop hydrochloric acid; after the reaction is completed, let stand to separate layers, wash the organic phase, dry, and evaporate the organic solvent to obtain product A4;
[0152] (42) Preparation B4: Diethyl 3,4-dimethoxybenzylphosphonate
[0153] A4 is mixed with triethyl phosphite, heated, refluxed, monitored by TLC, and processed af...
Embodiment 51-55
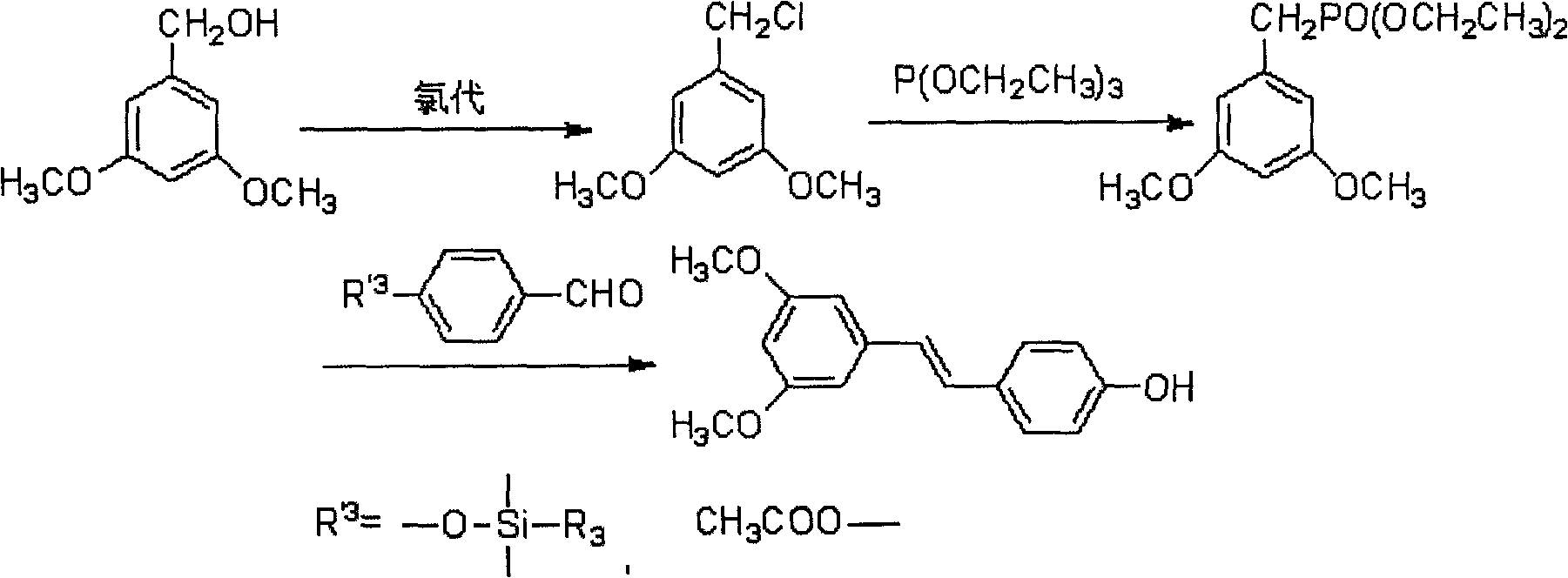
[0158] Example 51-55 Method 1 for the heterogeneous chlorination of hydrochloric acid to synthesize oxidized resveratrol
[0159]
[0160]
[0161] The method one of the above-mentioned heterogeneous chlorination of hydrochloric acid to synthesize oxidized resveratrol, its reaction route is as formula (VI):
[0162]
[0163] Formula (VI)
[0164] The first method for the heterogeneous chlorination of hydrochloric acid to synthesize oxidized resveratrol is carried out according to the following steps:
[0165] (51) Preparation A5: 3,5-dimethoxybenzyl chloride
[0166] Take 3,5-dimethoxybenzyl alcohol, add a solvent, and slowly drop hydrochloric acid; after the reaction is completed, let stand to separate layers, wash the organic phase, dry, and evaporate the organic solvent to obtain the product A5;
[0167] (52) Preparation B5: Diethyl 3,5-dimethoxybenzylphosphonate
[0168] A5 and triethyl phosphite were mixed, heated, refluxed, monitore...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com