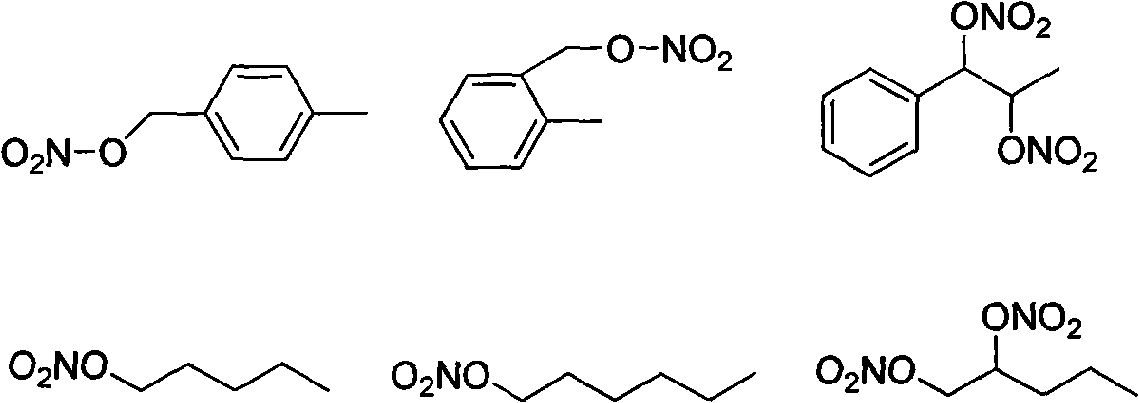
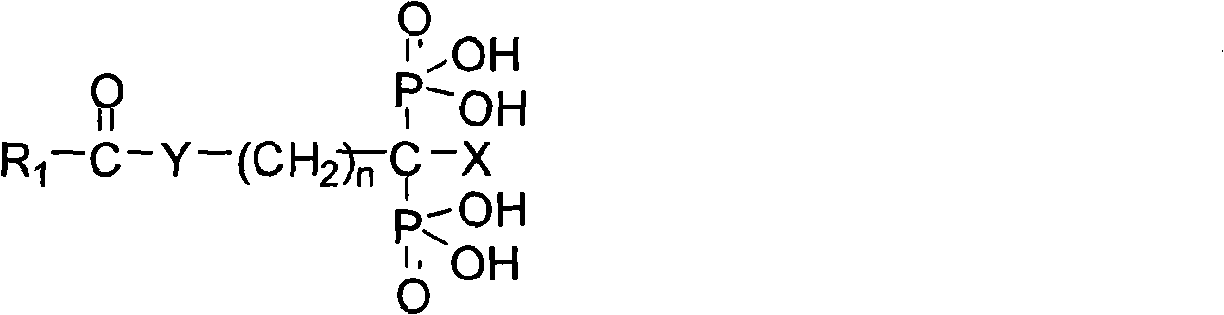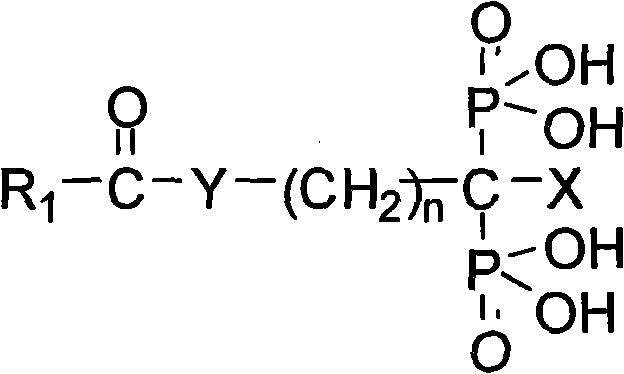Diphosphonic acid derivative containing NO donors, preparation method thereof and application thereof
A technology of bisphosphonic acid and derivatives, which is applied in the fields of chemical instruments and methods, drug combinations, and pharmaceutical formulations, can solve the problems of long synthetic routes and cumbersome preparation methods, and achieve strong practicability, simple and convenient synthetic methods, and reduced toxicity. side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: prepare NMBBDP
[0034] According to the synthetic route of previous NMBBDP, the specific preparation method of NMBBDP is as follows:
[0035] 1.1 Preparation of 4-nitroxymethylbenzoic acid (4)
[0036] In a 250ml three-necked flask, add 3g (0.02mol) p-cyanobenzyl chloride (2) and 50mLH 2 O, stir, heat to dissolve. Concentrated HCl (1.28mol, about 80ml) was added dropwise in three batches, refluxed for 5-6h, and cooled to room temperature. Let it stand overnight, adjust the pH value to about 3-4 with NaOH, filter, wash, and wash the solid with H 2 O was washed three times and dried in vacuo to give p-hydroxymethylbenzoic acid as a white solid (3).
[0037] Under cooling in an ice-water bath, in a 100ml three-neck flask, add 15ml of Ac 2 O and 7.6mlHNO 3 , stirred for 30min. Add 2g of p-hydroxymethylbenzoic acid prepared by the above steps dropwise and 15ml of Ac 2 The slurry composed of O, kept stirring during the dropwise addition and kept the tem...
Embodiment 2
[0047] Embodiment 2: Preparation of NHEBDP
[0048] 1.1 Dissolve 0.01mol 6-bromohexanoic acid (7) in 10ml of anhydrous acetonitrile, add dropwise to 3.4g of silver nitrate in 20ml of acetonitrile solution, maintain the temperature at 50°C, after the dropwise addition, raise the temperature to 70°C, and react for 6h. This reaction process should be protected from light. Cool, filter, remove the solvent by rotary evaporation under reduced pressure, add 8ml of water to the residue, extract with ethyl acetate (15mL×3), and dry over anhydrous sodium sulfate. Ethyl acetate was evaporated, and 50ml of ether was added to the residue, and filtered. The filtrate was distilled to remove the solvent to obtain light yellow liquid 6-nitrohexanoic acid (8).
[0049] 1.2 Add 10mmol of 6-nitrohexanoic acid (8) prepared in step 1.1 into the three-necked flask, add 20mL of SOCl dropwise under stirring 2 After the dropwise addition is completed, the temperature is slowly raised to 60-70°C unti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com