Catalytic oxidation method capable of recycling waste residues
A catalytic oxidation and waste residue technology, applied in chemical instruments and methods, oxidized water/sewage treatment, water/sludge/sewage treatment, etc. Cost, environmental protection, effect of reducing emissions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
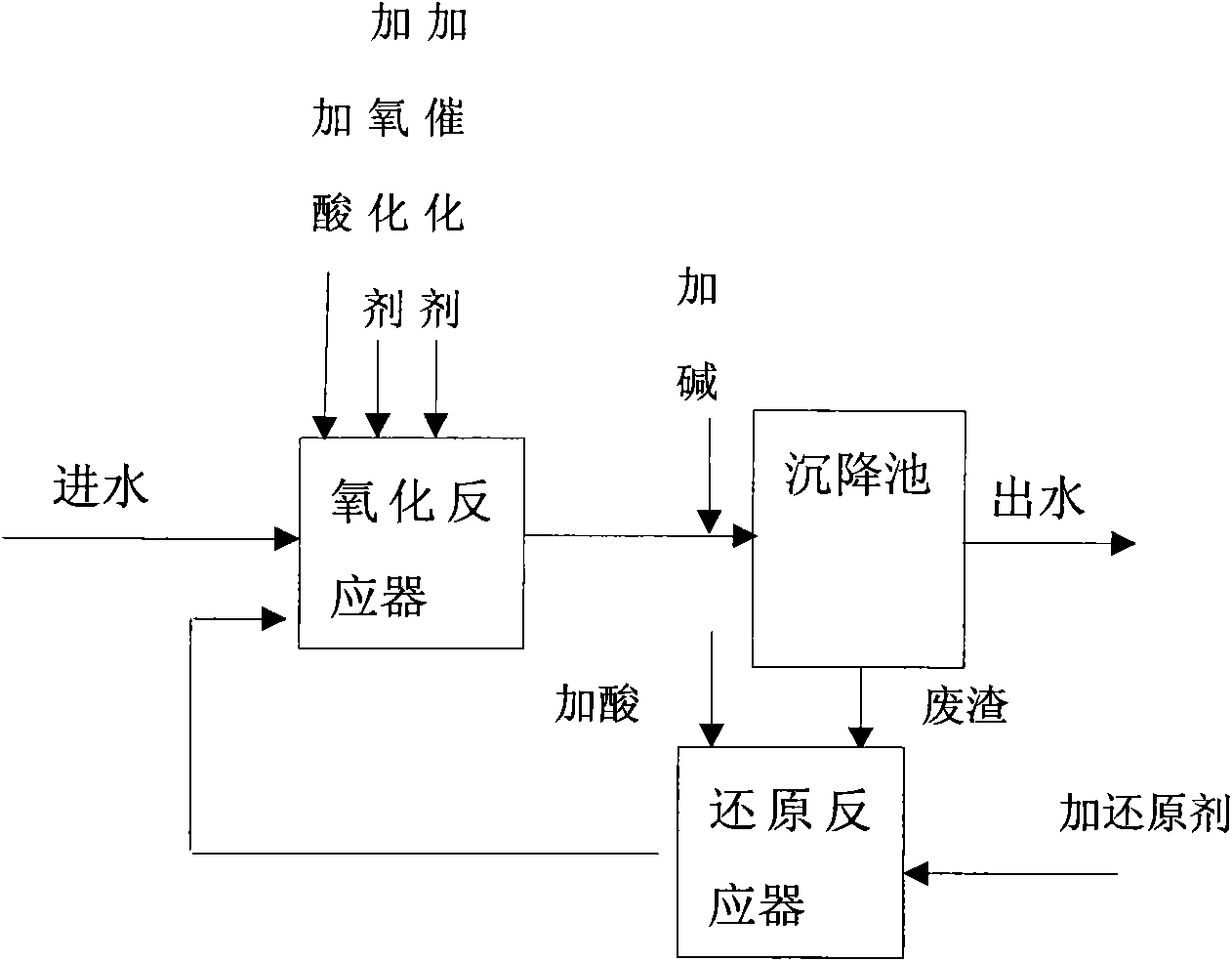
Image
Examples
Embodiment 1
[0026] To treat non-compliant wastewater discharged from a refinery, use H 2 O 2 As oxidant, Fe 2 SO 4 As a catalyst, iron filings as a reducing agent, and the acid and alkali used are concentrated H 2 SO 4 And NaOH. The operating conditions are determined as: H 2 O 2 / COD mass concentration ratio of wastewater 1:3, H 2 O 2 With Fe 2+ The molar concentration ratio is 1:2, reducing agent and Fe 3+ The molar ratio of the oxidation reaction tank is 1:1; the pH of the oxidation reaction tank is 3, the pH of the reduction reaction tank is 1, the pH of the sedimentation tank is 6.5, the residence time of the oxidation reaction tank is 30 minutes, the residence time of the sedimentation tank is 1 hour, and the residence time of the reduction tank is 20 Minutes; reducing agent and Fe 3+ The molar concentration ratio is 1:1. Table 1 shows the comparison of the data of the catalytic oxidation treatment method of the present invention and the traditional catalytic oxidation treatment metho...
Embodiment 2
[0031] To treat wastewater from a chemical fiber plant, use H 2 O 2 As oxidant, Fe 2 SO 4 Is a catalyst, sodium bisulfite is a reducing agent, and the acid base used is concentrated H 2 SO 4 And NaOH. The operating conditions are determined as: H 2 O 2 / COD mass concentration ratio of wastewater 1:10, H 2 O 2 With Fe 2+ The molar concentration ratio is 1:1, reducing agent and Fe 3+ The molar ratio of the oxidation reaction tank is 1:1; the pH of the oxidation reaction tank is 5, the pH of the sedimentation tank is 6.5, and the pH of the reduction reaction tank is 1.5; the residence time of the oxidation reaction tank is 30 minutes, the residence time of the sedimentation tank is 1 hour, and the residence time of the reduction tank is 30 minute. Table 2 shows the comparison of the data of the waste water treatment by the catalytic oxidation treatment method of the present invention and the traditional catalytic oxidation treatment method. The results in Table 2 show that the use...
Embodiment 3
[0036] To treat wastewater from a pharmaceutical factory, using sodium hypochlorite as the oxidant, Fe 2 SO 4 As a catalyst, sodium dithionite as a reducing agent, and the acid and alkali used are concentrated H 2 SO 4 And NaOH. The operating conditions are determined as: H 2 O 2 / COD mass concentration ratio of waste water 1:5, oxidant and Fe 2+ The molar concentration ratio is 1:3; the pH of the oxidation reaction tank is 3, the pH of the sedimentation tank is 6.5, and the pH of the reduction reaction tank is 1.2; the residence time of the oxidation reaction tank is 30 minutes, the residence time of the sedimentation tank is 1 hour, and the residence time of the reduction tank is 30 minute. Table 2 shows the comparison of the data of the waste water treatment by the catalytic oxidation treatment method of the present invention and the traditional catalytic oxidation treatment method. The results in Table 3 show that the waste residue and catalyst usage are significantly reduc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com